Metallic Bond Definition
Metallic bonding is a special type of bonding that holds the metals together in metal crystal. This bond is neither covalent nor ionic. Metals have tendency to give up electrons and none is their to accept it. There are several theories to explain this type of bonding, among them the electron sea model is most popular.
The electron sea model
Certain characteristics of metals are the reasons behind the metallic bond, these are:
- Metals have low ionization energy; that means metals can release their valence electrons readily. We know that ionization energy increase from left to right through the period and decreases down the group in periodic table.
- Metals have half filled orbitals. As for example the electron configuration of sodium is 1S22S22P63S1. Thus sodium has half filled s orbital.
Explanation of the electron sea model
Because of low ionization energy, the valence electrons of metals are loosely bonded within the atom. The similar half filled orbitals of adjacent metals can overlap with each other in metal crystal. The loosely bonded electrons can move freely in these orbitals to delocalize through out the whole crystal. Thus the positive metal ions stick together because of their electrostatic force between the neighboring positive ions for the delocalized electrons. The positive ions are immersed into the sea of electrons. This is why this model is known as the electron sea model.
Example
Sodium has one valence electron. This electron can delocalize through out the metal crystal to form the metallic bond. And the positive sodium atom immersed into the sea of delocalized electrons. The 3D model of sodium is as follows:
![Metallic Bond 18 By Benjah-bmm27 (Own work) [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/1/12/Sodium-crystal-3D-vdW.png)
Explanation of physical properties of metals by metallic bonding
The metallic bonding (electron sea model) can explain the physical properties of metals. Such as,
1. Ductile and malleable
Ductility is property of metals for what one can apply stress onto a metal to make it longer or wider without breaking.
Here (a) is brittle, (b) is partially ductile and (c) is completely ductile in nature. Malleability is another similar physical properties of metals where metals can be deformed under comprehensive stress. It can be measured by hammering or rolling a metal to form a sheet or to any other desired shape. The ability of this deformation depends on temperature. As for example, gold has high ductility and malleability. Thus nice shape of gold jewelry is available in market.
![Metallic Bond 20 By Eckhard Pecher (Own work) [CC BY 2.5 (http://creativecommons.org/licenses/by/2.5) or CC BY 2.0 de (http://creativecommons.org/licenses/by/2.0/de/deed.en)], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/d/da/Kanazawa_Gold_Factory.jpg)
These properties of metals can be explained by electron sea model of metallic bonding. When a metal is stressed by hammer or by any other way the internal structure of metal crystals remains unchanged. The electron sea and the positive ions adjust their position accordingly to restore the crystal structure of the stressed metal. This allows metal to be ductile and malleable.
2. Heat conductor
Metals are good conductor of heat. When one side of any metal is heated, the other end of the metal becomes hot. The electrons in the metallic bond in the heated part absorb heat and vibrate. It passes the energy to the next electrons and thus the heat energy carries till the end.
3. Electric conductor
Metals are also good conductor of electricity. We can get electric current, when electric voltage is applied to the metal wire. The mobile electrons in the metal can move freely to the specific direction when voltage applied. The best conductors of electricity are those metals which has minimum attraction to the valance electrons.
4. Photoelectric effect
Metals show photoelectric effect. When enough heat energy is applied to a metal, the electrons are emitted from the metal crystal. The free electrons in metal absorb required heat energy to overcome the attractive force within the metal crystal and thus emitted.
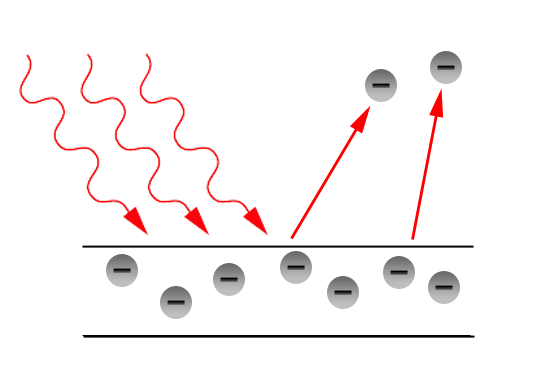
5. Reflection
Metals can reflect light. The electrons in a metal can absorb particular wavelength of light to jump to the higher energy level. When it comes back to original state, it emits the same wavelength of light. As the wavelength of incident light is same as emitted light, we see as reflection of light.
