Introduction
What is E-Z isomerism?
E-Z isomerism (also known as cis-trans isomerism or Geometric isomerism) is a type of stereoisomerism in which the same groups are arranged differently.
The general approach of the E-Z system is to observe the two groups at the end of each double bond. Then, analyze that the higher priority group at the one end of the double bond and the higher priority group at the other end of the double bond are on the same side (Z, from German zusammen = together) or the opposite sides (E, from German entgegen = opposite) of the double bond
A problem in naming Geometric E-Z isomers
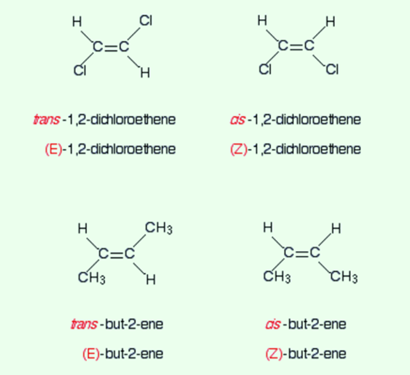
Consider a simple example of 1,2-dichloroethene. The geometric isomerism is given below:
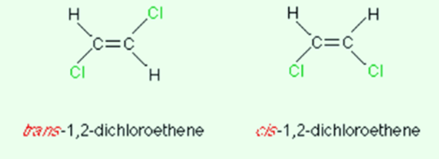
One can easily observe which one is cis and which is trans just in one glance. You must remember that trans means “across” and cis means “opposite”. This is a simple visual way of observing the two isomers than why do we need another system?
The problem arises when the compound gets more complicated. For example, are you able to name the isomers given below by using cis and trans?

The reason is that everything attached to the carbon-carbon double bond is different, the way they look doesn’t make obvious that they are being cis or trans to each other.
Working of E-Z system
We will use the second example to explain this system. Just look at each end of the double bond that is attached to each of them and give these two groups a priority according to a set of rules.
In the above example, there is bromine and fluorine on the left-hand side of the bond. It turns out that bromine has a higher priority than fluorine. At the right-hand side, chlorine turns out to be higher in priority than that of hydrogen.
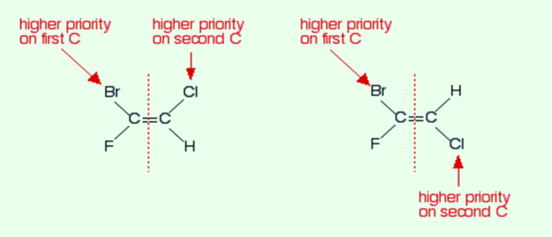
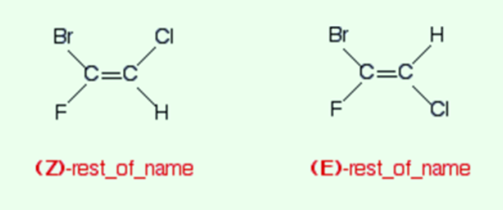
If the two groups having higher priorities are on the same side of the double bond than termed it as the Z-isomer. And if the two groups having higher priorities are on the opposite sides of the double bond, then termed it as E-isomer.
Hence the two isomers are
Rules for determining priorities
After the development of the system. The people named it as Cahn-Ingold-Prelog (CIP).
- The first rule for a simple case
Just have a look at the atoms that are attached directly to the carbon atoms at each end of the double bond-assuming the two and separately.
The atom has a higher atomic number will be given a high priority.
Let’s again have a look at the example that we have considered before too.

Now consider the first isomer and examine individually at the left-hand side and then the right-hand carbon atom. Now to declare the priorities just compare the atomic numbers of the atoms that are attached.

Notice that the atoms having the higher priorities are on the same side of the double bond. That will be declared as the Z-isomer.
Obviously at each end. the second isomer, however, it has the same atoms but this time the higher priority atoms are on opposite sides of the double bond. That is known as the E-isomer.
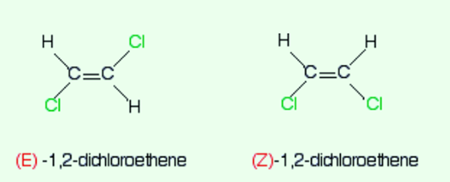
Consider another example of 1,2-Dichloroethene.

Now observe the priority of the two groups of the left-hand side isomer on the first carbon.
The atomic number of chlorine is higher than that of hydrogen and therefore has a high priority. This goes the same for the carbon atom present in the other isomers.
The first isomer will be declared as the E-isomer as the higher priority groups are on opposite sides of the bond. The other one will be declared as Z-isomer as the higher priority groups are on the same side.
Now consider an example of but-2-ene.
Here you will encounter a slight complication because here we have not got a single atom attached to the double bond but a group of atoms.
This is not a problem just focus on the atom that is directly attached to the double bond here the carbon in the CH3 group.
In this case, you can ignore the hydrogen atom in the CH3 group entirely. But in complicated groups, one just has to worry about the atoms that are not directly attached to the double bond.
The given below is one of the isomers of the but-2-ene.

The CH3 group is considered the high priority because the carbon atom here has an atomic number of six as compared with an atomic number of one for the hydrogen which is also attached to the carbon-carbon double bond.
The isomer that is drawn above has the two higher priority groups on opposite sides of the double bond. hence, the compound is E-but-2-ene.
- Minor addition to the rule to allow for isotopes
Deuterium, an isotope of hydrogen has a relative atomic mass number of 2. It has one proton and therefore yet has an atomic number of one. You will see that it isn’t the same as that of the atom of an atom of ordinary hydrogen and hence these two compounds given below are geometric isomers.

The deuterium and hydrogen have the same atomic number, therefore, they have the same priority on this base. In such a case the one having the higher relative atomic mass has a higher priority. So in such isomers, the chlorine and the deuterium are counted among the higher priority groups on each end of the double bond.
Hence the left-hand isomer in the last diagram is the E-form and the right-hand one is the Z-form.
- Extended rules to more complicated molecules
Consider the following complicated example to find out whether it is a Z or E isomer by applying some additional rules
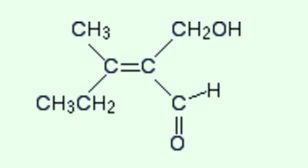
Just observe the left-hand end of the molecule. What is attached directly to the carbon-carbon double bond?
A carbon atom is attached directly to the bond in both of the attached groups. These two atoms will have the same atomic number and hence the same priority. So this will not help for sure.
In these types of cases, you now observe what is directly attached to those two carbons but without counting the carbon of the double bond and then compare the priorities of these next lot of atoms.
In simple cases, you can do this in your head but sometimes it is necessary to write the attached atoms down and enlist them having the high priority atom first. This will help in comparing. For example
In the CH3 group
The atoms attached to the carbon are H H H.
In the CH3CH2 group
The atoms which are attached directly to the carbon of the CH2 group are C H H.
In the second list, the C is written first because it has the highest atomic number
Now compare the two lists atom by atom. The first atom in each list is an H in the CH3 group and a C in the CH3CH2 group. The carbon will have a higher priority because of having a higher atomic number. Therefore, this gives the CH3CH2 group a higher priority than the CH3 group.
Now just notice the other end of the double bond. the extra thing that describes is that if you are having a double bond, just count the attached atom twice. The given below is the structure again.
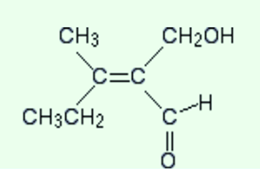
Here again, the atoms which are attached directly to the carbon-carbon double bond are both carbons. Therefore, we need to look at what is attached to those carbons.
in the CH2OH group
the atoms attached directly to carbon are O H H
in the CHO group
the atoms attached directly to the carbon are O O H.
Don’t forget that the oxygen is counted twice because of the carbon-oxygen double bond. in both of the lists, the first oxygen is written because of having a higher atomic number than hydrogen.
So what is the priority of the two groups? Oxygen is the first atom in both of the lists but that will not help. Now observe the next atom in both the lists. In the CH2OH group, it’s hydrogen and in the CHO list, it’s oxygen.
The oxygen will have a high priority and this gives the CHO group a higher priority than the CH2OH group.
The isomer is, therefore, a Z-form because the two higher priority groups i.e. CH3CH2 group and the CHO group, are both on the same side of the bond.
Can cis- and trans- be easily translated into Z and E?
Consider the example of 1,2-dichloroethene and but-2-ene cases.
But this rule doesn’t work every time. Just look at this uncomplicated molecule.
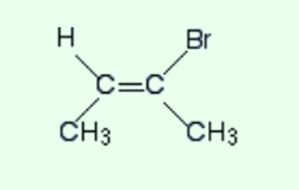
This is a cis-isomer. There are two CH3 groups on the same side of the double bond. but also figure out the priorities on the right-hand end of the double bond.
The two atoms that are directly attached are carbon and bromine. Bromine has a high atomic number so it will have a higher priority on that end. On the other end, the CH3 group will have a high priority.
This shows that the two high priority groups are on the opposite sides of the double bond so this is an E-isomer and not a Z-. Note that you can never convert these systems into the other direction.
