Geometric Isomers Definition
Geometric isomerism is a kind of stereoisomerism. It is also known as cis-trans isomerism or E-Z isomerism. Geometric isomerism occurs due to the restricted rotation about carbon-carbon double bonds or carbon-carbon single bonds in cyclic compounds.
Geometric isomers are the stereoisomers which differ from each other in the arrangement of groups with respect to the double bond or ring structure. Two types of notification is possible for these geometric isomers:
- cis-trans notation
- E-Z notation
1. cis-trans notation
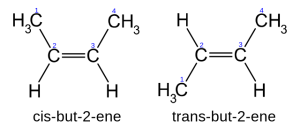
In cis-isomer the two similar groups stay on the the same side of the double bond and in trans-isomer they stay in opposite side. For example, 2-butene has two isomers cis and trans. In cis-isomer two methyl groups and two hydrogen groups stay in the same side of the double bond, while in trans-isomer they stay in opposite side.
2. E-Z notation
When none or most of the groups attached to the double bond are not same then geometric isomers are notified or named with E or Z. For the notification of this type of isomers we find the highest priority atom (highest atomic number) attached to each double bonded C. If two highest priority atoms stay in same side of the isomer that is designated as Z and if they are in opposite side they are designated as E.

As for example, 1-bromo-1-fluropropene has two geometric isomers. In Z-1-bromo-1-fluoropropene we can see that bromo- has higher priority or higher atomic number (35) than fluoro (9) which are attached to C-1. Again carbon has higher atomic number (6) than hydrogen (1) which are attached to C-2 of this compound. As the highest priority atoms carbon (from -CH3 group) and bromine attached to these two carbons are on the same side, this compound is notified as Z. On the other hand in E-1-bromo-1-fluoropropene the highest priority atoms C and bromine are in opposite side, thus it is named as E-isomer.
Geometric isomerism in cyclic compounds
In a cyclic compound the rotation between carbon-carbon single bond is restricted. Thus geometric isomerism is also possible for this type of compounds if two different groups are attached to each carbon. For example, geometrical isomerism is not possible for 1.1-dimethylcyclopropane but it is possible for 1,2-dimethylcyclopropane.
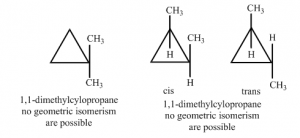
There are two isomers of 1,2-dimethylcyclopropane. One is cis-isomer where two methyl groups are on same side and other is trans-isomer where two methyl groups are on different side.
Comparison of physical properties of two isomers
Geometric isomers often differ in their physical properties. This is because of the shape of the isomers and the overall dipole moment.
Such as sometime they differ in boiling point. For example the boiling point of cis and trans isomers of 1,2-dichloroethene are 60.3 oC and 47.5 oC.
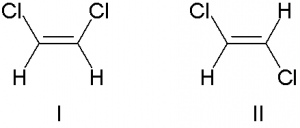
In cis-isomer, the presence of two dipole bond (C-Cl) gives a overall molecular dipole. This results intermolecular dipole-dipole forces. For this force cis isomer have more boiling point than trans-isomer where the two dipole bond (C-Cl) is cancelled out because of their position in opposite direction.
Summary
- Geometric isomerism occurs due to the restricted rotation about carbon-carbon double bonds or carbon-carbon single bonds in cyclic compounds.
- In cis-isomer the two similar groups stay on the the same side of the double bond and in trans-isomer they stay in opposite side.
- If two highest priority atoms stay in same side of the isomer that is designated as Z and if they are in opposite side they are designated as E.
- In a cyclic compound if the rotation between carbon-carbon single bond is restricted and two different groups are attached to each carbon geometric isomerism is possible in similar manner.
- Geometric isomers often differ in their physical properties. This is because of the shape of the isomers and the overall dipole moment.
