INTRODUCTION
What is organic chemistry?
Organic chemistry involves the study of compounds that contains carbon mainly. Simple compounds like carbonates, carbon dioxide, and carbon monoxide is an exception. Carbon is singled out because of its extraordinary chemical diversity by any other chemical element. Its diversity is based on the following:
- Carbon atom forms a very strong bond with other carbon atoms
- Carbon atoms form a very strong bond with atoms of other elements.
- Carbon atoms form a large number of covalent bonds.
History
Organic chemistry involves the study of compounds containing carbon. Organic chemistry is a branch of science that is considered to be originated in 1685 with the publication by Lemery of a chemistry book that classifies substances according to their origin as mineral, vegetables, or animal. Compounds that are derived from plants and animals were termed as organic chemistry and those derived from nonliving sources were termed as inorganic.
Until 1828, it was considered that the organic compounds cannot be formed other than by living plants and animals. This was named as the vital-force theory. People believed that very seriously that the development of organic chemistry almost stopped. In 1828, a scientist named Wohler accidentally found that if ammonium cyanate (an inorganic compound) is heated, it causes it to change into urea, a compound that is considered organic.
This discovery dealt a death blow to the vital-force theory, and by 1850 modern organic chemistry became were established. Today approximately 13 million organic compounds are known. Many of these are the products of synthetic chemistry and similar compounds are not known in nature. Around 70,000 organic chemicals are used commercially.
Elements
All organic compounds contain carbon in combination with one or more elements. The hydrocarbons contain only hydrogen and carbon. A great many compounds contain carbon, hydrogen, and oxygen, and they are considered to be the major elements. Naturally occurring compounds in minor elements are Sulphur, nitrogen, phosphorous and sometimes halogens and metals. Synthetic compounds may contain besides, a wide variety of other elements.
Characterization
As organic compounds often exist as mixtures, diversified techniques have also been developed to evaluate the purity, especially important being chromatography techniques such as HPLC and gas chromatography. Traditional methods of separation include distillation, crystallization, and solvent extraction.
Different chemical tests were characterized historically on organic compounds, called “wet methods”, but spectroscopic or other computer-based methods of analysis has taken place of such tests. Some important analytical methods are given as follows
Nuclear magnetic resonance (NMR) spectroscopy is the most commonly used technique that permits a complete assignment of atom connectivity and even stereochemistry by using correlation spectroscopy. The primary constituent of organic chemistry-hydrogen and carbon- exist naturally with NMR-responsive isotopes, respectively 1H and 13C.
Elemental analysis: To determine the elemental composition of a molecule this destructive method is used.
Mass spectrometry describes the molecular weight of a compound and its structure with the help of fragmentation patterns. With the help of high-resolution mass spectrometry, we can identify the exact formula of a compound. The old spectrometry was limited to neutral molecules that exhibit volatility to some extent but in the latest ionization techniques, we can get the mass spec of any organic compound.
Crystallography- it is useful for determining the molecular geometry of a single crystal of material is available.
Properties
The physical properties of organic compounds include both quantitative and qualitative features. Quantitative features involve melting point, boiling point, and refraction index. Qualitative features include odor, consistency, color, and solubility.
- Melting and boiling properties
Almost all of the organic compounds melt and boil. On the other hand, inorganic compounds generally can be melted, most of them do not boil and tend to degrade. In the old days, the melting and boiling points explain the crucial information on the purity and identity of the organic compounds.
The melting and boiling points correlate with the polarity of the molecules and their molecular weight. Few organic compounds, especially the symmetrical ones elevated that they evaporate without melting. For example, para-dichlorobenzene. Most of the organic compounds may not remain stable at very high temperatures like above 300 C, but some exceptions may also exist.
- Solubility
Neutral organic compounds tend to be hydrophobic i.e. they are less soluble in water than in organic solvents. Exceptions include the organic compounds that are ionizable groups also the low molecular weight alcohols, amines and carboxylic acids where hydrogen bonding occurs.
The organic compounds tend to dissolve in organic solvents. These solvents can either be pure substances like ether or ethyl alcohol or mixtures such as paraffinic solvents like white spirits and petroleum ethers.
The solubility in the different solvents depends upon the solvent type and on the functional groups if present in the solution.
Nomenclature
The names of the organic compounds are either systematic that follows logically a set of rules while the non-systematic follows various traditions. There are more than 6million known organic compounds. Therefore, the nomenclature is very important.
Find the longest carbon chain in the molecule. This will tell us the base of the name
| No. of C atoms | Name |
| 1 | meth-ane |
| 2 | eth-ane |
| 3 | prop-ane |
| 4 | but-ane |
| 5 | pent-ane |
| 6 | hex-ane |
| 7 | hept-ane |
| 8 | oct-ane |
| 9 | non-ane |
| 10 | dec-ane |
Determine the principal functional group and its position
| Principal functional group | Formula | Ending becomes |
| Alkane | C-C | -ane |
| Alkene | C=C | -ene |
| Alkyne | C≡C | -yne |
| Alcohol | -OH | -anol |
| Aldehyde | -CH=O | -anal |
| Ketone | >C=O | -anone |
| Carboxylic acid | -COOH | -anoic acid |
We can indicate the position by numbering the carbons in the main chain where necessary. Alkanes do not require any position indication as there is no functional group and aldehydes and acids too because they are terminal functional groups.
Ancilliary functional groups are written in alphabetical order, with their position at the beginning of the name.
| Ancilliary functional group | Formula | prefix |
| Methyl | -CH3 | Methyl |
| Ethyl | -C2H5 | Ethyl |
| Propyl | -C3H7 | Propyl |
| Butyl | -C4H9 | Butyl |
| Pentyl | -C5H11 | Pentyl |
| Hexyl | -C6H13 | Hexyl |
| Heptyl | -C7H15 | Heptyl |
| Octyl | -C8H17 | Octyl |
| Nonyl | -C9H19 | Nonyl |
| Decyl | -C10H21 | Decyl |
| Fluorine | -F | Fluoro |
| Chlorine | -Cl | Chloro |
| Bromine | -Br | Bromo |
| Iodine | -I | Iodo |
| Amine | -NH2 | Amino |
| Hydroxyl | -OH | Hydroxyl |
| Cyanide | -CN | Cyano |
| Benzyl | -CH2C6H5 | Benzyl |
| phenyl | -C6H5 | phenyl |
Structural drawings
Organic molecules are expressed commonly by drawings or structural formulas or combinations of drawings and chemical symbols. The line-angle formula is simple and clear. In this type of system, the endpoints and intersections of each line indicate one carbon and hydrogen atoms can either clearly or assumed to be present as tetravalent carbon.
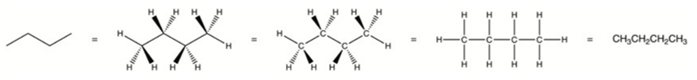
Classification of organic compounds
- Functional groups
The concept of a functional group plays a significant role in organic chemistry as it classifies the structures and also predicts the properties. A functional group is a molecular module and its reactivity is assumed within limits to be the same in a variety of molecules. A functional group may have a decisive influence on the chemical and physical properties of organic compounds. Molecules are classified based on their functional groups
- Aliphatic compounds
The aliphatic hydrocarbons are further classified into three groups of homologous series according to their state of saturation
- Alkanes (also termed as paraffin): aliphatic hydrocarbons in absence of double or triple bonds i.e. C-C, C-H single bonds.
- Alkenes (also termed as olefins): aliphatic hydrocarbons having one or more double bonds i.e. di-olefins or poly-olefins.
- Alkynes (also termed as acetylenes): aliphatic hydrocarbons having one or more triple bonds.
The remaining group is classified according to the number of functional groups present. Such compounds can be a straight-chain, branched-chain or cyclic. The characteristics are affected by the number of branching, i.e. octane number or cetane number in petroleum chemistry.
Saturated and unsaturated compounds both exist as cyclic derivatives. The most stable ring has five of six carbon atoms.
- Aromatic compounds
Aromatic hydrocarbons consist of conjugated double bonds. This means that all carbon atoms in the ring are sp2 hybridized which helps in the stability. The most common and important example is benzene as it is one of the simplest and stable aromatics.
- Heterocyclic compounds
In the presence of heterocyclic atoms, the characteristics of the cyclic hydrocarbons can be changed, which can exist either substituent attached externally to the ring (exocyclic) or as a member of the ring itself (endocyclic). In the case of later, the ring is termed as heterocyclic. The examples are pyridine and furan.
The heteroatom of heterocyclic molecules includes oxygen, Sulphur, or nitrogen. Heterocycles are usually found in aniline dyes and medicines.
- Polymers
Carbon has an important property that it readily forms chains or networks that are linked by carbon-carbon bonds. This linking process is known as polymerization, while the chains or networks are termed as polymers. The source compound is called a monomer.
Polymers are divided into two main classes
- Synthetic polymers
- biopolymers
Synthetic polymers are those which are manufactured artificially and are generally known as industrial polymers while biopolymers occur in a natural environment or you can say without the interference of humans.
- Biomolecules
Biomolecular chemistry is a major branch of organic chemistry which is studied by biochemists. Most of the complex multi-functional group molecules are important in living organisms. Some of them are long-chain biopolymers which include DNA, peptides, RNA and polysaccharides i.e. starches in animal and cellulose in plants. In addition to it, animal biochemistry contains many small molecules intermediates which help in the production of energy through the Krebs cycle and results in isoprene, which is the most common hydrocarbon in animals.
Organic reactions
Organic reactions are chemical reactions that involve organic compounds. Most of these reactions are related to functional groups. The general theory of these reactions involves the analysis of such properties as electron affinity, bond strengths, and steric hindrance.
The basic reaction types include; addition reactions, redox reactions, elimination reactions, pericyclic reactions, substitution reactions and rearrangement reactions. A simple example of a substitution reaction can be written as
Nu– + C-X → C-Nu + X–
where X can by any functional group and Nu is a nucleophile.
