Introduction
- What is a catalyst?
A catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction.
- What is catalysis?
Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst.
A very small amount of catalyst is required to alter the reaction rate.
What is a catalyzed reaction?
A catalyzed reaction is typically used to accelerate the rate by which specific chemistry is to proceed.
Generally, the role of the catalyst is to provide an alternative, low energy pathway for a reaction. For this to happen, the catalyst interacts with a reactant and forms an intermediate compound. This intermediate is temporary in that after it forms, it breaks apart and leaves the original catalyst species unchanged.
Types of catalyzed reactions
There are two major types of catalyzed reactions
- Heterogeneous Catalyzed Reaction
- Homogeneous Catalyzed Reaction
A heterogeneously catalyzed reaction occurs when the catalyst and the reactant exist in two different phases i.e. a solid catalyst in the presence of a reactant in solution.
A homogeneously catalyzed reaction occurs when the catalyst and the reactant are in the same phase i.e. when the catalyst and the reactants are dissolved in the same solution.
Now let’s have a look that what a phase is?
What is a phase?
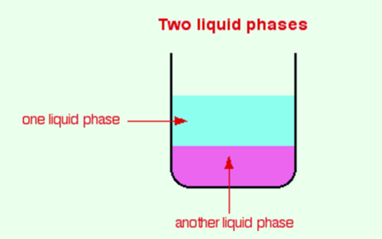
Consider a mixture. Now observe a mixture carefully. You will see that there is a boundary between two of the components which indicates that the substances are in different phases. A mixture that contains a solid and a liquid consists of two different phases while a mixture that contains various chemicals in a single solution consists of only one phase because there is no apparent boundary between them.
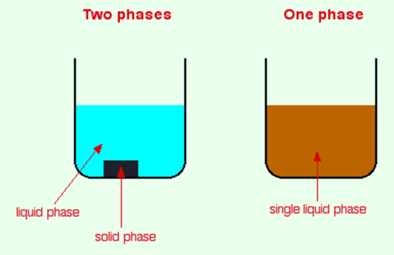
You might think that the why phase differs from the term physical state (solid, liquid or gas). It may include solids, liquids, and gases but is a bit more general. It can also be applied to the liquids that do not dissolve into each other but forms a boundary between the two liquids i.e. oil and water.
Explanation
Heterogeneous catalysis
This type includes the use of a catalyst in a different phase from the reactants. The common examples involve a solid catalyst with the reactants as either liquids or gases.
Working principle of a heterogeneous catalyst
Majority of the examples of the heterogeneous catalysis go through the same stages
One or more than one of the reactants are adsorbed on to the surface of the catalyst at active sites.
Adsorption is the process in which atoms, ions or molecules from different substances like solid, liquid or gas adhere to a surface of the adsorbent. On the other side absorption is a process in which a fluid is completely dissolved by an absorbent.
An active site includes that part of a surface which is particularly good at adsorbing things and also help them to react.
There is some kind of interaction between the surface of the catalyst and the reactant molecules which makes them more reactive. This might involve an actual reaction with the surface or some weakening of the bonds in the attached molecules.
The reaction occurs and at this stage, both of the reactant molecules might attach themselves to the surface or one might get attached and hit by the other one that moves freely in the gas or liquid.
The product molecules are desorbed.
Desorption generally means the breakdown of the product molecules. This makes a space available at active sites for the new molecules to come, attach and to react.
A catalyst is considered as a good catalyst that is adsorbed to the reactant molecules strong enough that they react but not that much stronger than the product molecules stick more or less permanently to the surface.
For example, silver is not considered a good catalyst because it does not attach strongly with the reactant molecules while tungsten too is not considered a good catalyst as it adsorbs too strongly with the reactant molecules.
Metals like platinum and nickel are considered as a good catalyst because they absorb strongly to hold and activate the reactants but not that much strongly that the products can’t break away.
Example of heterogeneous reactions
The hydrogenation of a carbon-carbon double bond
the simplest example of this type of reaction is between ethene and hydrogen in the presence of a nickel catalyst.
CH2 = CH2 + H2 Ni as catalyst CH3CH3
In actual, this is a useless reaction, because you are converting the very useful ethene into the relatively useless ethane. However, the same reaction will occur with any compound that contains a carbon-carbon double bond.
One of the important industrial use is in the hydrogenation of vegetable oils to make margarine, which also involves reacting a carbon-carbon double bond in the vegetable oil with hydrogen in the presence of a nickel catalyst.
Ethene molecules are adsorbed on the surface of the nickel. There is a breakdown of a double bond between carbon atoms and hence the electrons are used to bond it to the nickel surface.
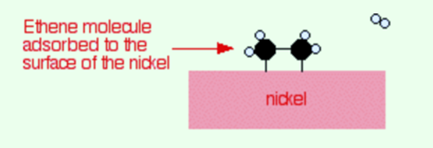
There is adsorption of hydrogen molecules on to the surface of the nickel. When this takes place, the hydrogen molecules break into atoms. These can move around on the surface of the nickel.
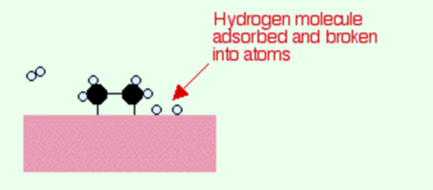
If there is a diffusion of hydrogen atoms close to one of the bonded carbons, the bond between the carbon and the nickel is replaced by one between the carbon and hydrogen.
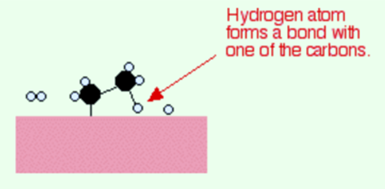
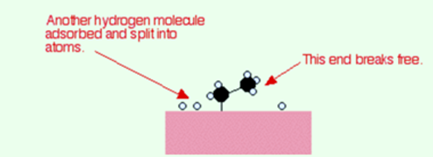
Now that end of the original ethene breaks free of the surface, and eventually, the same thing will occur at the other end.
As earlier, one of the hydrogen atoms forms a bond with the carbon and that end also breaks free. Now there is a space on the surface of the nickel for new reactants molecules to go through the whole process again.
Catalytic Converters
Catalytic converters change poisonous molecules like carbon monoxide and different nitrogen oxides in car exhaust into less harmless molecules like carbon dioxide and nitrogen. The expensive metals are used like rhodium, platinum, and palladium as they are a heterogeneous catalyst.
The metals are deposited on a ceramic honeycomb as thin layers. This increases the surface area and minimizes the use of metal.
The reaction between carbon monoxide and nitrogen monoxide can be written as
2CO + 2NO Pt/ Pd / Rhconverts to 2CO2 +N2
Homogeneous Catalytic Reactions
In these reactions, the catalyst is in the same phase as the reactants. Generally, everything will be present as a gas or contained in a single liquid phase.
Examples of homogeneous catalytic reactions
The reaction between persulphate ions and iodide ions
Persulphate ions are very strong oxidizing agents while iodide ions are very easily oxidized to form iodine. In water, these reactions are very slow.
The reaction equation is given below
S2O82- + 2I– converts to 2SO42- + I2
The reaction requires a collision between the two negative ions. The catalyzed reaction avoids that problem fully. The catalyst used here can either be an iron II or iron III ions that are added to the same solution.
Another good example of the use of the transition metal compounds as catalysts because of having an ability to change oxidation state.
First, we will take iron II ions as a catalyst but after some time you will observe that it doesn’t matter at all whether you use iron II or iron III ions
The persulphate ions oxidize the iron II to iron III ions. During this reaction, the persulphate ions are reduced to sulphate ions.
S2O82- + 2Fe2+ converts to 2SO42- + 2Fe3+
The iron III ions are much strong that they oxidize the iodide ions to iodine easily. In this process, they are reduced back to iron II ions again.
2Fe3+ + 2I– converts to 2Fe2+ + I2
In an overall reaction, both of these individual stages involve a collision between positive and negative ions. This will be a much successful reaction in the presence of a catalyst other than in an uncatalyzed reaction.
Other examples of Catalytic Reactions
- The halogenation of benzene
Benzene reacts with chlorine or bromine in the presence of a catalyst. The catalyst used can either be aluminum chloride or iron.
Strictly talking about iron is not a catalyst, because it gets permanently changed during the reaction. It reacts with chlorine or bromine to form iron III chloride, FeCl3 or iron III bromide, FeBr3.
2Fe + 3Cl2 converts to 2FeCl3
2Fe +3Br2 converts to 2FeBr3
These compounds act as a catalyst and act exactly the same as aluminum chloride in these reactions.
- The reaction with chlorine
The reaction between benzene and chlorine in the presence of either aluminum chloride or iron gives chlorobenzene.
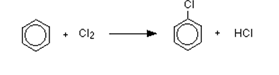
- The reaction with bromine
The reaction between benzene and bromine in the presence of either aluminum bromide or iron gives bromobenzene. Generally, iron is used because it is not very expensive and easily available.
C6H6 + Br2 converts to C6H5Br + HBr
- The Friedel-crafts alkylation of benzene
Alkylation includes the replacement of a hydrogen atom on a benzene ring by an alkyl group like methyl or ethyl. This represents another example of the use of aluminum chloride as a catalyst. Benzene is allowed to react with chloroalkane in the presence of aluminum chloride as a catalyst. The below equation shows the reaction by using a methyl group but any other alkyl group can be used in the same manner.
Substituting a methyl group gives methylbenzene, which was once known as toluene.
C6H6 + CH3Cl converts to C6H5CH3 + HCl
References
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_(Inorganic_Chemistry)/Catalysis/Examples/Examples_of_Catalysis/5._Examples_of_Other_Catalytic_Reactions_in_Organic_Chemistry
- https://en.wikipedia.org/wiki/Catalysis
- https://www.mt.com/my/en/home/applications/L1_AutoChem_Applications/L2_ReactionAnalysis/Catalytic-Reactions.html
- https://www.chemguide.co.uk/physical/catalysis/introduction.html
