Introduction
Aluminium is a light weight metal with silvery white appearance [1]. It is denoted by symbol “Al”. In Purest form, the aluminium metal is bluish- white in colour.
Position in Periodic table
It belongs to group 13 of the periodic table. This group has 3 electrons in their outermost shell. Elements of this group including aluminum are metallic in nature. Among all elements, Boron and aluminum have greater commercial value than others in the same group [2].
Periodic table information
- Atomic number
Atomic number of aluminium is 13.
- Atomic weight
Atomic weight of aluminium is 26.98 amu.
- Electronic configuration
Aluminium has 13 electrons and 14 neutrons. The proton number is also 13. The electron configuration for Aluminum is 1s22s22p63s23p1. Aluminium has 3 electrons in valence shell [3] [6].
- Oxidation states
Three oxidation states of aluminium are +3, +1 and+2. In compound Al2 O3, Aluminium has oxidationstate of +3 [4].
- Occurrence
Aluminium rarely occurs free in nature. Mostly, it binds with oxygen to form Alumina. This bound form of aluminium is present in nature. However it is among most abundant metals on earth. It is usually present in Bauxite and Cryolite form. These are basically Aluminium Silicates [5]. More forms of aluminium present in nature are Micas, Feldspar, Vermiculite, Gibbsite, garnet and emerald.
- Earth crust:
Aluminium is third most occurred metal present in earth crust. It covers almost 7% to 8 % of earth’s crust [7] [8].
- Discovery:
Aluminium was already being used by ancient Greeks and Romans and was given name alumina. It was further discovered and called as “alum” [9]. Davy identified its existence but it was not successfully isolated by him.
- Nomenclature:
Aluminum is given this name from a word of Latin origin “Alumen or alum”. Guyton de Morveau gave it name “alumina” in 1761. After that, it was given name “Alumium” by Humphrey Davy in 1807. After some time, he renamed it as “Aluminum” [10]. After some time conforming with named of a lot of other elements, it was renamed as Aluminium. This name was further adopted by IUPAC and is still used all over the world except in North America. So both spellings “Aluminum” and “Aluminium” are considered correct in periodic table.
- Extraction of aluminium:
It was first isolated by Hans Oersted in 1825. He heated and mixed anhydrous Alumnium Chloride with potassium amalgam to get minute quantity of aluminium.
It was then isolated in 1827 by Fredrick Wohler. He heated and mixed Aluminium chloride with potassium in a platinum container to obtain Aluminium.
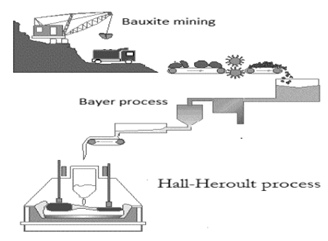
Bauxite is the substance having greater amount of naturally occurring Alumnium and impurities present in bauxite can be removed from aluminium. 90 % of bauxite is used for aluminium production. Bauxite is hydrated oxide of aluminium. Impurities present in bauxite are silica, ferric oxide and clay. Bauxite is used for extraction of aluminium [11]. It is a complicated process. When this process is performed at industrial scale, it gives maximum yield of aluminium.
Karl Josef Bayer (Austria) in 1888 patented the method to produce aluminum and the process known as Bayer process. Another process was invented in 1886 by Charles Martin Hall and Paul Louis Toussaint Héroult and the process known as “Hall-Herolt Process”.Bauxite is first dissolved in molten sodium hydroxide at 1200 ºC and is then precipitated with carbon dioxide after that, it is dissolved in cryolite at 950 ºC and electrolyzed by Bayer process [12].
Chemical properties [13].
- Reaction with air:
Alumnium don’t necessarily react with oxygen in air due to the presence of a protective oxide coating on metal surface. Still, if some surface remains unprotected, then it will react with oxygen giving aluminium oxide as product.
4Al (s) + 3O2 (l) → 2Al2O3(s)
- Reaction with acids
Aluminium is rapidly dissolved in concentrated hydrochloric acid.
2Al(s) + 3H2SO4(aq) → 2Al3+(aq) + 2SO42-(aq) + 3H2(g)
- Reaction with Halogens:
Alumnium metal reacts with halogens to form halides. This is usually vigorous reaction.
2Al(s) + 3Cl2(l) → 2AlCl3(s)
- Reaction with bases:
Alumnium reacts with bases by following reactions.
2Al(s) + 2NaOH(aq) + 6H2O → 2Na+(aq) + 2[Al(OH)4]– + 3H2(g)
Physical properties [14]
- Density
Aluminium has a density of 2.7 g/cm3
- Melting point
It has melting point of 660 degree Celsius.
- Boiling point
Aluminium has boiling point of 2470 degree Celsius.
- Isotopes:
Alumnium has around 22 isotopes. Among these isotopes 27 Al is stable.
- Crystal structure
Solid Aluminium has crystal structure of face centre cube.
- Specific gravity
Specific gravity of aluminium is 2.7.
- Light weight
Atomic weight of aluminium is 26.9.
- Corrosion resistance:
Self protecting and coating process helps aluminium to attain maximum corrosion resistance.
- Electrical conductivity
Aluminium is excellent conductor of electricity. This is the reason it in found in various wirings. Especially it is used for long distance transmission of electricity. The electrical conductivity of aluminium is about twice of the copper [15].
- Thermal conductivity
Alumnium has relatively higher thermal conductibbity. There is about 50% -60% of increased conductivity in aluminium a compared to that of copper.
- Reflectivity :
The reflective nature of aluminium with other alloys make it upcoming good source for mirror polishing.
- Ductility:
Aluminium in its purest form is very ductile. This is reason it cannot be used in purest form for construction purpose. Addition of silicon or iron makes it perfect for use as building material.
- Impermeable:
Alumnium inform of sheets is totally impermeable to heat and light, which makes its good option for food storage and transportation.
- Recycling:
Materials made up of aluminium can be easily recycles to yield aluminium back. This recycling loop makes it good source material to be used in everyday life [16].
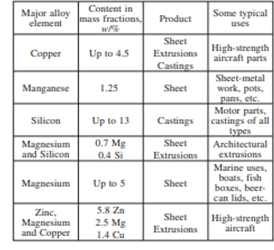
Alloys of aluminium:
When any element or group of elements are added to Alumnium in order to improve the physical properties, then they are known as alloys of Alumnium. Aluminium is usually used in alloy form as pure aluminium is not strong. For this purpose, some alloys are produced to improve the strength of aluminium products but keeping in mind that light weight nature of metal remains same. Some alloys are shown in table below:
Uses of aluminium
Physical properties of aluminium make it possible to be used in variety of purposes. Some of these uses make it really important in our everyday life [17].
- Utensils
Alumnium is a good conductor of heat and light in weight. These two qualities make it a good choice for kitchen utensils [18].
- House materials
House building materials such as windows are made up of Alumnium.
- Automotive industry
From last many years, aluminium has been used in automotive industries. Major applications in this field includes: Auto body, chassis and other structural components. Light weight vehicles made up of aluminium parts, with maximum corrosion resistance can help reducing fuel consumption. It is easier to clean this metal and that’s reason it is used in automotive industry. No surface protection is required if aluminium is used.
- Marine applications
With the advancement in aluminium industry and with invention of new aluminium alloys, it has now been used in marine applications. This is due to the fact, aluminium has higher corrosion resistance. So, for marine travel, this quality is of much importance, along with being light in weight [19].
- Food packaging
Alumnium is being used in food packaging such as cans and in aluminium foil for kitchen use.
- Sporting goods
Some sport equipments are nowadays made up of aluminium. Example is: Golf carts and bicycles.
- Furniture
Some furniture such as beds is also made up of aluminium.
- In Drugs
Alumnium in form of aluminium hydroxide is used as antacid drugs.
- Printing plates
Alumnium in form of thin sheets is used for printing plates.
- Paper Making
Aluminium Sulphate has been used in paper industry. Paper manufacturers use it as binder of dyes and glues.
- Nano technology
Anodic aluminium oxide (AAO) is compound of aluminium with really great properties. It has a unique structure which makes it available to be used in nanotechnology.
- Aviation industry:
Wright brother, while discovering the first aeroplane, also used aluminium as major component of aircraft engine. This is due to its light weight property. Nowadays due to its favourable physical properties, it has been extensively used in aviation industry. Aluminium, when interacts with oxygen, makes a protective coating which prevent metal from corrosion.
Alumnium is widely used metal. It has a great number of applications depending upon its physical and chemical properties.
REFERENCES
- https://www.britannica.com/science/aluminumhttps://rsc.org/periodic-table/element/13/aluminiumhttps://www.saburchill.com/chemistry/visual/atoms/013.htmlhttps://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Moduleshttp://science.marshall.edu/castella/chm448/elements1.pdf(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_pBlock_Elements/Group_13%3A_The_Boron_Family/Z%3D013_Chemistry_of_Aluminum_(Z%3D13)www.aalco.co.ukRoesky, Herbert & Kumar, Shravan. (2005). Chemistry of Aluminum (I). Chemical communications (Cambridge, England). 32. 4027-38. 10.1039/b505307b.Guyton de Morveau, L. B. (1782), “Mémoire sur les dénominations chimiques, la necessité d’en perfectionner le système et les règles pour y parvenir”, Observations sur la Physique, 19: 370–382.Sir Humphry Davy. Elements of chemical Philosophy. Part 1. Volume 1 (1812) https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p Block_Elements/Group_13%3A_The_Boron_Family/Z%3D013_Chemistry_of_Aluminum_(Z%3D13)/Aluminium_MetallurgyProduction of primary aluminium H. Kvande, in Fundamentals of Aluminium Metallurgy, 2011https://www.webelements.com/aluminium/chemistry.htmlhttps://www.asminternational.org/documents/10192/1849770/06787G_Sample.pdf https://www.thoughtco.com/aluminum-or-aluminium-3980635Corrosion of Aluminum and Aluminum Alloys (#06787G) Editor(s): J.R. Davishttps://www.aluminum.org/industries/production/recyclingm. j. freiría gándara: aluminium: the metal of choice. materiali in tehnologije / materials and technology 47 (2013) 3, 261–265 https://aluminiuminsider.com/aluminium-alloys-in-shipbuilding-a-fast-growing-trend/https://opportunitymuse.com/5-benefits-using-aluminum-cookware.https://www.azom.com/article.aspx?ArticleID=4193
