INTRODUCTION OF SULPHURIC ACID
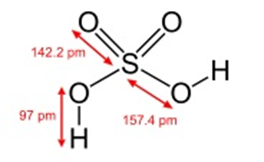
Sulphuric acid is a chemical compound (H2SO4). It is odorless, colorless, extremely corrosive, oily liquid and sometimes it is called oil of vitriol. Concentrated sulphuric acid is a weak acid because of its poor electrolytes, at room temperature, a little bit of it is dissociated into ions. But on the other hand, it is also considered as the king of chemicals. Its boiling point is too high. It is known as an oxidizing agent. When we cool the concentrated sulphuric acid it does not react with such metals like iron and copper. When in the hot condition the sulphuric acid reacts with most of the metals and non-metals like sulphur and carbon. It is soluble in water because of its syrupy liquid texture.
MOLECULAR FORM OF SULPHURIC ACID
HISTORY OF SULPHURIC ACID
Sulphuric acid was discovered by Jabar ibn Hayyan in the 8th century and later on, in 9th century, the physician and alchemist Ibn Zakkriya AL Razi studied the properties of sulphuric acid and obtain the substance by dry distillation of minerals including iron (II) sulfate heptahydrate FeSO4.7H2O and copper (II) sulfate pentahydrate CuSO4.5H2O.
When these compounds are heated they were converted into iron oxide and copper oxide, giving off water and sulphur trioxide, when these compounds are combined suphuric acid is formed. Later on, it was famous in Europe. The Medieval European alchemist named the sulphuric acid “oil of vitriol” because it was prepared by roasting ‘green vitriol’ Iron (II) sulfate in an Iron retort (Device used to obtain the substance by dry distillation). In 1746, by the Lead chamber process the sulphuric acid was produced, invented by John Roebuck and soon it was improved by many others. The process created by john roebuck approached a 65% concentration.
Later purification to the lead chamber by the French chemist Joseph Louis Gay-Lussac and British chemist Johan Glover improved concentration to 78%. The process produced by green vitriol was expensive. In 1830 the contact process was developed by Peregrine Philips in England. The contact process was very economical for producing sulphur trioxide and sulphuric acid. Today this method is used to produce sulphuric acid.
USES OF SULPHURIC ACID
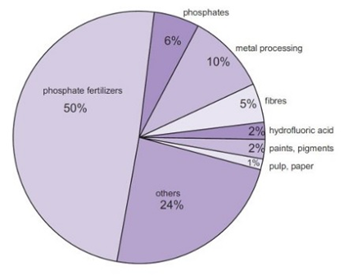
INDUSTRIAL USES
Sulphuric acid is a strong mineral acid. Most of the sulphuric acid used in the industry and it is also known as the industrial compound. Sulphuric acid is a good indicator because of its industrial strength.
- The major use of sulphuric acid is in the industry.it is used in different industries in different ways. The most important use of sulphuric acid in the industry is in the production of phosphoric acid by the wet method (phosphoric acid is produced by reacting sulphuric acid naturally occurring phosphate rock).
AGRICULTURAL USE
- The major usage of sulphuric acid is in the fertilizers e.g. superphosphate of lime and ammonium sulphate. Almost 60% of the sulphuric acid is consumed for fertilizers particularly superphosphates, ammonium phosphate, and ammonium sulphate. It is also used in potato farming.
BLEACHING AGENT
- In the manufacture of chemicals like synthetic detergents, drugs, dyes, pigments.
OIL REFINING
- It is used to refine the petroleum and to wash the impurities out of gasoline.
MEDICAL PROCESSES
- Skin infections can be treated by sulphuric acid. It is used in skin ointments. It is used in the treatment of canker sores; it is the basic ingredient of the ointment named Debacterol.
- Sulphuric acid is used in the making of various types of drugs. Like Chemotherapy, it is used to cure cancer.
CLEANING PROCESS
- Home usage of sulphuric acid is to drain cleansers but it should be handled carefully because it is a very dangerous chemical.
STEEL MANUFACTURING
- Sulphuric acid is used in the manufacturing of copper and zinc and in cleaning the surface of the steel sheet. This cleaning process is known as “pickling”. It converts the steel into a thin layer which is used to make cans.
AUTOMOBILE BATTERIES
- It is used in motor vehicles. Concentrated sulphuric acid is the electrolyte in the lead-acid storage battery.
RAYON
- Rayon has medium strength. Rayon is used in the textile industry. Textile rayon is made from cellulose fibers derived from the wood. Cellulose fiber is dissolved in the solution. This solution contains cellulose fiber and Tetra Amine Copper which produce thick blue liquid. This thick blue liquid is injected into sulphuric acid to form Rayon fibers.
- It is also used in medical bandages.
PAPER BLEACHING
- Bleaching of paper pulp is done with concentrated sulphuric acid. It is also used in paper sizing.
WATER TREATMENT
- Sulphuric acid is used to bring the ph level of wastewater back to normal.it separates dissolved waste from the water during this process.it eliminates the virus and bacteria from the water.
MANUFACTURING OF VETERINARY DRUGS
- Veterinary drugs are the medicines that are used to give animals to treat them from injury, disease, and pests in livestock. Sulphuric acid is used in the manufacturing of veterinary drugs to cure the animal of disease.
EXPLOSIVES PRODUCTION
- Concentrated sulphuric acid is used in the manufacturing of explosives. It acts as a catalyst in making any kind of explosives that contain nitrate.
SYNTHESIS OF PLASTICS
- Sulphuric acid is used as a catalyst in the synthesis of plastics.
DETERGENTS
- It is used in making soaps and detergents. Castor oil is used as a source of vegetable oils.it reacts with sulphuric acid to form a detergent.
MAKING OF PAINTS
- Concentrated sulphuric acid is a viscous liquid which absorbs the water vapors from the air and becomes a solution. It is observed that sulphuric acid is being used for removing paints from the rubber surface.
JET FUEL
- A small amount of sulphuric acid reacts with water to form H2SO4. It does not dissolve directly to the jet fuel. It is highly corrosive.
LEATHER
- Animal skin is converted into the leather by using sulphuric acid along with other chemicals. This process is called the pickling process.
PICKLING PROCESS
CARBON SUGAR
- Carbon sugar is formed by the reaction of sugar with the dehydration of sulphuric acid. It removes the water from the sugar and converts into thick black tube.it is also called Carbon Snake
ANNUAL PRODUCTION OF SULPHURIC ACID
| WORLD | 270 million tons |
| CHINA | 74 million tons |
| US | 37 million tons |
| INDIA | 16 million tons |
| RUSSIA | 14 million tons |
| MORROCO | 7 million tons |
| PAKISTAN | 4 million tons |
SAFETY
LABORATORY HAZARDS
Sulphuric acid has a highly exothermic reaction with water. The process of making concentrated sulphuric acid is very dangerous.it has corrosive properties. If it contacts with skin, then immediately go for first aid treatment. The contaminated cloth must be removed from the skin and underlying skin should be washed.
INDUSTRIAL HAZARDS
Sulfuric acid is non- flammable. The dispersal of acid aerosols and gaseous sulfur dioxide is an additional hazard of fires involving sulfuric acid. Water should not be used as an extinguishing agent because of the risk of further dispersal of aerosols. Exposure to aerosols may lead to eye irritation and respiratory tract.
REFERENCES
https://en.wikipedia.org/wiki/Sulfuric_acid
https://www.cs.mcgill.ca/~rwest/wikispeedia/wpcd/wp/s/Sulfuric_acid.htm
https://www.encyclopedia.com/science-and-technology/chemistry/compounds-and-elements/sulfuric-acid
