Introduction to amines
Amines are organic functional group where one of more hydrogen atoms of ammonia (NH3) are replaced by alkyl or aryl groups. When alkyl group is attached with ammonia it is known as alkylamines and when aryl group is attached it is known as arylamine. When both groups are present in amine, it is called alkylarylamine.
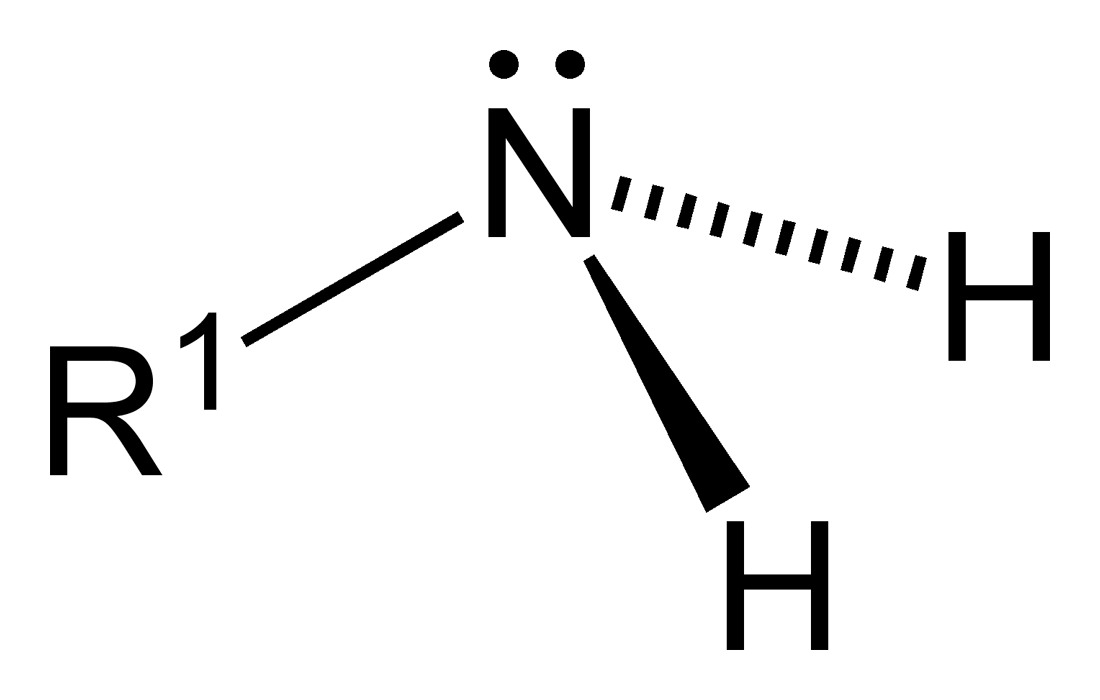
Classification of amines
According to the number of alkyl groups attached to the nitrogen atom, amines are classified as:
1. Primary (10) amines
When only one alkyl group is attached to the nitrogen atom of an amine by replacing one hydrogen atom, that is called primary (10) amine.
2. Secondary (20) amines
When two alkyl groups are attached to the nitrogen atom of an amine, that is called secondary (20) amine.
3. Tertiary (30) amines
When three alkyl groups are attached to the nitrogen atom of an amine, that is called tertiary (30) amine.

Structure of amines
Nitrogen has 5 valence electrons and the electron configuration of nitrogen is 1s2 2s2 2px1 2py1 2pz1. In amine nitrogen and carbon both are sp3 hybridized. Three sp3 hydrid orbitals of nitrogen are half filled and one them are filled with electron which can not take part in bond formation. So according to VSEPR theory the amines are trigonal pyramidal shape where the nitrogen atom is at the apex and the lone pair of electrons stay just above it. Each C-C σ bond is formed by overlapping the two sp3 hybrid orbitals of two atoms. To form N-H bond the sp3 hybrid orbital of nitrogen is overlapped with the s orbital of hydrogen atom.

Nomenclature of amines
The following rules are applied while naming amines:
1. For primary amines, the name of the alkyl group is prefixed to amine. Such as,
| Name | Structure |
| Methylamine | CH3-NH2 |
| Ethylamine | CH3CH2-NH2 |
| n-Propylamine | CH3CH2 CH2-NH2 |
2. If more than one identical alkyl groups are attached to the nitrogen atom, then the prefix di- or tri- is added to the name of alkylamine. As for example,
| Name | Structure |
| Dimethylamine | (CH3)2-NH |
| Triethylamine | (CH3CH2)3-N |
| Trimethylamine | (CH3)3-N |
3. When the alkyl groups of amines are not identical the name of the alkyl groups are added alphabetically. Such as,
| Name | Structure |
| Ethylmethylamine | CH3-NH-CH2CH3 |
| Ethylisopropylamine | CH3CH2-NH-CH(CH3)2 |
4. When the amine is too complex to name following the rules above, it is named using the IUPAC system. In this system, the longest chain of carbon is selected and the amine is considered as a substituent. The numbering of longest chain starts in such a way that the amine group gets the lowest number. The number and name of the other substituent are also added with the name of amines.
| Name | Structure |
| 5-Methyl 3-aminohexane | CH3-CH2-CH(NH2)-CH2-CH(CH3)2 |
| 2-Amino-2-methylpropane | C(CH3)3-NH2 |
Physical properties of amines
Boiling point
1. Boiling point of amines are greater than the boiling point of corresponding alkanes because of the presence of hydrogen bond between two amines as well as van der Waals dispersion forces and dipole-dipole interactions.
2. The order of the boiling point of three types of amines- primary, secondary and tertiary are:
BP of Primary amine > BP of Secondary amine > BP of Tertiary amine.
This is because of the more alkyl groups gets on the way of hydrogen bonding. Less hydrogen bonding and at least boiling point for tertiary amines.
Solubility
1. Unlike most of the other organic compounds, amines are soluble in water due to the formation of hydrogen bonding between amine and water molecule.
2. The solubility of amine decreases with the increase of longer chain of alkyl group or the number of alkyl groups attached to the amines, as the increased alkyl groups gets on the way of hydrogen bonding between water and amine molecule. Thus the order of the water solubility of amines is:
Primary amine > Secondary amine > Tertiary amine.
