INTRODUCTION
What is carbocation?
A carbocation is a molecule that has a carbon atom that bears three bonds and a positive formal charge. Generally, the carbocations are unstable because they do not have eight electrons that satisfy the octet rule.

Classification of Carbocation
A carbocation is classified into three basic categories
- Primary carbocation
- Secondary carbocation
- Tertiary carbocation
- Primary carbocations
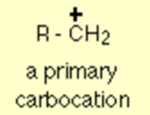
In a primary carbocation, the positive charge which is carried by carbon is only attached to one other alkyl group.
By using the symbol R for an alkyl group, a primary carbocation can be written as follows
Few examples of primary carbocation are given below:
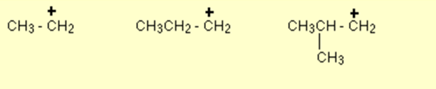
Observe that it doesn’t matter how complicates the attached alkyl group is you just need to count the number of bonds from the positive carbon to other carbon atoms. In the examples given above, there is only one such link.
- Secondary carbocation
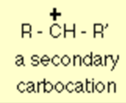
In a secondary carbocation, the positively charged carbon is attached to two other alkyl groups, which may be different or the same.
The general formula of a secondary carbocation can be written as given below, where R and R’ indicates the alkyl groups which may be the same or different.
Few examples of secondary carbocation are given below:
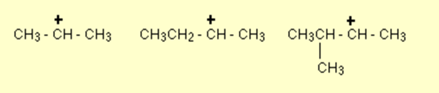
- Tertiary carbocation
In a tertiary carbocation, the positively charged carbon is attached to three alkyl groups, which may be any combination of the same or different.
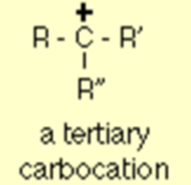
A general formula of tertiary carbonation is given below where R, R’ and R” are alkyl groups and it may be different or same.
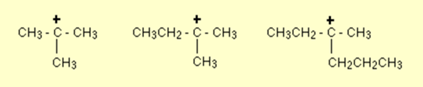
Few examples of tertiary carbonation are given below
Tertiary Carbocation stability
Generally, Carbocations are unstable and relatively hard to form. Usually, they can’t be isolated from a reaction as they immediately react to fill their empty p orbital. As they are electron deficient so when the attaching electron donates groups like alkyl groups, the carbonation will help to stabilize the carbonation. General stability order shows very clearly that tertiary carbocation is the most stable among all, as it is surrounded by three other carbon atoms that share the burden of its positive charge.
The electron pushing effect of alkyl groups
Maybe you are familiar with an idea that bromine is more electronegative than that of hydrogen, therefore in the H-Br bond, the electrons are grasped closer to the bromine than the hydrogen. a bromine atom which is attached to a carbon atom would have exactly the same effect- the electrons being pulled towards the bromine end of the bond. the bromine has a negative inductive effect.
The alkyl group will act exactly the opposite and rather than drawing electrons towards themselves, they tend to push electrons away. This means that the alkyl group becomes slightly positive and the carbon to which they are attached becomes slightly negative. So the alkyl group will have a positive inductive effect.
This is occasionally shown as, for example
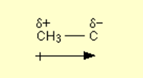
The arrow indicates that the electrons are being pushed away from the CH3 group. The plus sign on the left-hand end of it indicates that the CH3 group is becoming positive. The symbols δ+ and δ- simply support that idea.
The importance of spreading charge around in making ions stable
According to a rule-of-thumb if a charge is too localized (all concentrated on one atom) the ion will be much stable than if the charge is spread out over several atoms.
Applying that to carbocations of all three kinds
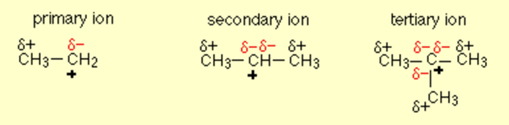
You will notice that the electron pushing effect of the CH3 group is to put a more and more negative charge on the positive carbon as we move from primary to secondary to tertiary carbocations. This effect is obviously to cut down that positive charge.
At the same time, the area that is around the various CH3 groups is becoming somewhat positive. Then the net effect is that the positive charge is spread out over more and more atoms as we move from primary to secondary to tertiary ions.
The more we spread the charge around, the more stable the ion becomes.
The stability of tertiary carbocation in term of energy
If we talk about secondary carbocations being more stable than primary ones, what exactly do we mean? Here, we are actually talking about the energetic stability-in energy ladder which is given below shows that the secondary carbocations are lower in the energy ladder than the primary ones.
It means more energy is required to make primary carbocation than a secondary one. If we have a choice between making a secondary ion or primary one, definitely to make secondary carbocations will be much easier.
Similarly, in an energy ladder, tertiary carbocation is lower than secondary ones, so secondary carbocations will require more energy to form than tertiary carbocations. If we will be given a choice of making a tertiary or secondary one, we will definitely go for tertiary carbocations.
This has important implications in the reactions of unsymmetrical alkenes.
Factors that stabilize tertiary carbocations
There are three main factors that increase the stability of tertiary carbocations
- By increasing the number of adjacent carbon atom: methyl is the least stable carbocation <primary<secondary<tertiary (the most stable carbocation).
- The adjacent pi bonds which allow the p-orbital carbocation to be a part of a conjugated pi-system (delocalization through resonance)
- Adjacent atoms with lone pairs
