Introduction to E-Z nomenclature
E-Z notation is used to name geometric isomers. It is an extension of cis-trans isomer. When none or most of the groups attached to the double bond are not same then in IUPAC system geometric isomers are notified or named with E or Z. For the notification of this type of isomers we find the highest priority atom (highest atomic number) attached to each double bonded Carbon. If two highest priority atoms stay in same side of the isomer that it is designated as Z and if they are in opposite side they are designated as E.

Notification of E-Z isomers
When two identical groups are on the same side of the double bond, that is called cis-isomer. And when they are on opposite side of the double bond, that is called trans-isomer. When there are no identical groups attached with two carbons of the double bond, then the notification is done using the IUPAC system. All geometric isomers can be notified using this system. The naming of isomers using this system follow few rules, these are:
Rule 1:
First we need to find out the atom which has highest atomic number between the two atoms attached to the each carbon of the C=C double bond. Then we need to see if the atoms with highest atomic number stays on the same side of the double, then it is called Z-isomer and if they are on opposite side then it is notified as E-isomer. As in the following molecule 1-bromo-1-chloro-2-fluro-2-iodoethene has two isomers E and Z. In first one the number 1 carbon contains bromo and chloro groups. We know that the atomic number of bromine is 35 which is higher than the atomic number of chlorine (the atomic number of chlorine is 17). Again the atomic number of iodine is 53 which is higher than the atomic number of fluorine (the atomic number of fluorine is 9). So if the two highest priority groups bromine and iodine attached to two carbon atoms are on the same side then it will be Z-isomer and if they are on opposite sides, that will be E-isomer.
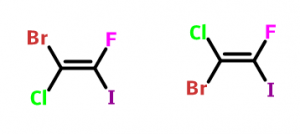
Now the figure above is showing that in first one (left) the two highest priority groups bromine and iodine are in opposite side, thus it is E-isomer. And in second one (right) the two highest priority groups are in same side, thus it is Z-isomer.
Rule 2:
When the two atoms attached to the same carbon are identical, then the atomic number of next atom will be taken under consideration and will be selected as highest priority accordingly. As for example, 1-chloro-2-methyl-1-butene has two isomers. Hydrogen and chloro groups are attached in number 1 carbon. Among these, chlorine atom has highest priority over the hydrogen atom. Because the atomic number of chlorine is 17 and hydrogen is 1. Again Ethyl and methyl groups are attached to number 2 carbon. The carbon atoms of both groups are attached with the double bonded carbon atom. As they are identical, now we need to consider the next atoms attached to these two carbons of methyl and ethyl groups. In methyl group carbon is attached to three hydrogens and in ethyl group the carbon atom is attached with one carbon and two hydrogen atoms. So the carbon in ethyl group is attached with highest atomic number that means another carbon atom. So the ethyl group is the highest priority over the methyl group. Now two highest priority groups attached with two double bonded carbon (C=C) are chloro and ethyl groups. For first left molecule in following figure, we will name as E-isomer as the two highest priority groups chloro and ethyl are on opposite sides. And for second right one, we should name as E-isomer as they are on the same side.

