Hydrolysis
It means cleavage of chemical bonds of compound through the addition of water. During hydrolysis reaction with pure water is slow and usually not used, but ester is catalyzed by dilute acid like dilute hydrochloric acid and dilute sulphuric acid [1].
For example: H+ (aq)
CH3COOCH2CH3 + H2O ⇋ CH3COOH + CH3CH2OH
Ethyl ethanote ethanoic acid ethanol
Another example hydrolyzing methyl proponate
H+(aq)
CH3CH2COOCH3 + H2O ⇋ CH3CH2COOH + CH3OH
Methyl propanoate propanoic acid methanol
The reactions are reversible, use excess amount of water to make the hydrolysis as complete as possible, the water comes from dilute acid so ester mix with excess of dilute acid.
Hydrolysis through dilute alkali
Sodium hydroxide is used to hydrolyzing ester, ester are heated with dilute alkali like sodium hydroxide. The reaction is one way and the products are easily separated.
CH3COOCH2CH3 + NaOH → CH3COONa + CH3CH2OH
Ethyl ethanoate sodium ethanoate ethanol
Hydrolyzing methyl propanote in the same way
CH3CH2COOCH3 + NaOH → CH3CH2COONa + CH3OH
Methyl propanote sodium propanote methanol
Ester
Ester was first reported by German chemist Leopold Gmelin in first half of 19th century, class of organic compound, structurally an alkoxy (OR) group is attached to the carbonyl group. They react with water and produced alcohols and organic or inorganic acids. In reactions alkoxy group is replaced by another group like hydrolysis which means splitting with water. It is catalyzed by either through base or an acid like hydrochloric acid or sulfuric acid. It is the reverse process of esterification (preparation of an ester). R may be alkyl, aryl or may be H [2]. It can be prepared through reactions of acid anhydride with alcohol and reaction of carboxylic acid salts with alkyl halides. Esters can be converted into another type of ester through transesterified process, in which through reaction with an alcohol, a carboxylic acid or third ester in the presence of catalyst. Saponification is the process in which ester hydrolysis is carried out in the presence of alkalies, it is used in the preparation of soaps from fat or oils, also helpful for quantitative estimation of ester.
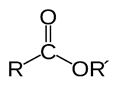
Ester
Nomenclature of Ester
The ester first word is derived from alkyl group and second word is from carboxylate group of the carboxylic acid.
Preparation of Ester
Ester are most commonly prepared through the reactions of carboxylic acid, acid chlorides and acid anhydrides with alcohol [3].
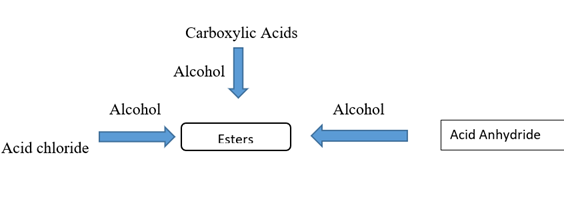
Synthesis of Ester
Acid – Catalyzed Esterification
The reaction of carboxylic acid and alcohol forms ester. The catalytic amount of a strong inorganic acid like sulfuric acid (H2SO4), Hydrochloric acid (HCl) and H3PO4 while organic acid include benzenesulpohonic or ρ- toluenesulphonic acid are used because they are soluble in organic solvent and they are also used as catalyst without adding additional amount of water
In the process of acid hydrolysis ester is heated with large amount of water which contain a strong acid catalyst. This reaction is reversible and does not go for completion like esterification.
For example: butyl acetate reacts with water to produce acetic acid and 1- butanol.
CH3COOCH2CH3CH3 + H2O ⇋ CH3COOH + CH3CH2CH2CH2OH
Butyl acetate water Acetic acid 1- Butanol (butyl alcohol)
Esters derived from an Acid chloride and an Alcohol
Acid chloride and alcohol reacts with each other in the presence of a base like pyridine or Na2CO3 to make esters, the weak base is neutralize the liberated HCl.
Pyridine
Butanoyl chloride + Ethanol → Ethyl butanoate + hydrochloric acid
Ester derived from an Acid Anhydride and an Alcohol or a Phenol
Acid anhydride react with phenol or alcohol via nucleophilic acyl substitution to produce ester in the presence of a catalyst like strong acid (H2SO4) or a weak base (pyridine) or through heating.
Hydrolyzing complicated esters for soap making
Hydrolyzing big ester are commonly found in animals and vegetables fats or oils. Large esters are heated with concentrated sodium hydroxide produce a salt of carboxylic acid and octadecanoic acid (stearic acid). The salt is very important by-product which is helpful in cleaning. An alcohol is also produced called as propane-1,2,3-triol (glycerol).
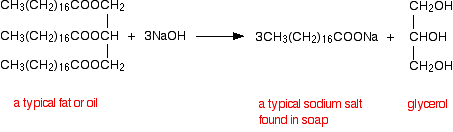
The alkaline hydrolysis of an ester called as saponification.
Uses and Application
- Presence of ester in fruits and flower is responsible for flavor and fragrance like: isopentyl acetate in bananas, methyl salicylate in wintergreen and ethyl butyrate in pineapple [5].
- Volatile ester is used in the preparation of synthetic flavor, perfumes and cosmetics.
- Ester in volatile in nature also used as solvent in lacquers, paints and varnishes, for example ethyl acetate and butyl acetate.
- In animals and plants waxes are secreted, they are ester formed from long chain carboxylic acid and long chain alcohol.
- Low volatile liquid esters are used as softening agents in resins and plastics.
- As a polymer, Polymethyl methacrylate is an ester, it is a glass substitute sold as Lucite and Plexiglas.
- Phosphate esters are important on industrial point of view, they are used as solvent, plasticizers, flame retardants, gasoline, oil additives and as insecticides.
- Sulfuric and sulfurous acid ester are used in the preparation of dyes and pharmaceuticals.
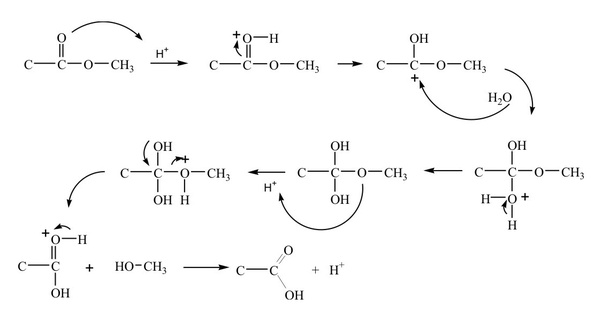
Mechanism of the acid catalyzed hydrolysis of esters
First step
Activate the ester because an acid/base reaction, weak nucleophile and a poor electrophile is present. So the protonation of the ester carbonyl make it more electrophilic [4].
Second step
The oxygen (O) of water function as nucleophile attack on the electrophilic C in the C=O and electron moving towards oxonium ion, making tetrahedral intermediate.
Third step
Deprotonate the oxygen which is came from the water molecules and neutralize the charge.
Fourth step
There is a need to make the –OCH3 leave, but first protonation and then convert it into good leaving group.
Fifth step
With the help of electron of adjacent oxygen “push out” the leaving group called neutral methanol molecule.
Sixth step
Now deprotonation of the oxonium ion make carbonyl C=O in the carboxylic acid product and reproduce the acid catalyst.
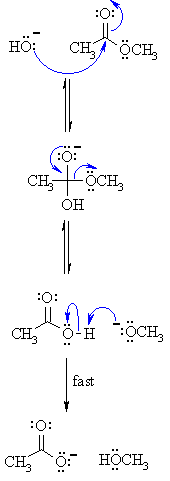
Mechanism of base hydrolysis of Esters
First step
The hydroxide nucleophiles attack on the electrophilic C of the ester compound C=O, they break the double bond and make the tetrahedral intermediate.
Second step
The intermediate collapses and again form the C=O results in the loss of alkoxide leaving group, leading to the carboxylic acid.
Third step
Equilibrium is formed where alkoxide, RO– behave as a base deprotonating the carboxylic acid, RCO2H.
Applications of Ester Hydrolysis
- Sodium acetic acid is a kind of salt. When water is added to sodium acetic acid derivation at that point the compound separate into sodium particles and acetic acid particle. Acetic acid the secondary particle combine with hydrogen and make acidic corrosive.
- Alkyl halides used in refrigerants as CFCs (chlorofluorocarbons). When water is added in the alkyl halide substance it is changed in to another form called liquor, in this form it is secure for earth [6].
- Hydrolysis of sucrose which is disaccharide and called as table sugar breakdown into fructose and glucose which is used for different purpose.
References
- https://www.chemguide.co.uk/organicprops/esters/hydrolysis.html
- https://chem.libretexts.org/Courses/Eastern_Mennonite_University/EMU%3A_Chemistry_for_the_Life_Sciences_(Cessna)/15%3A_Organic_Acids_and_Bases_and_Some_of_Their_Derivatives/15.09_Hydrolysis_of_Esters
- https://profiles.uonbi.ac.ke/andakala/files/upc_213_esters.pdf
- http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch20/ch20-3-3-1.html.
- https://www.britannica.com/science/ester-chemical-compound
- https://byjus.com/chemistry/ester-hydrolysis/