Introduction
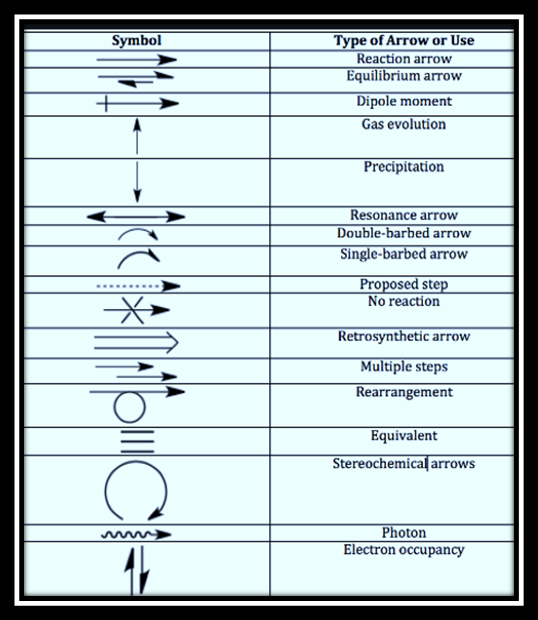
There are different types of arrows we meet in organic chemistry. Before explaining curly arrows, let’s have a glance at these types individually.
- The forward arrow (→), which is also known as reaction arrow. The goal of this arrow is to indicate an action. There is no hard and fast rule about what is supposed to write above or below an arrow but reagents are mostly written above and solvents tend to go below.
- The Equilibrium arrow (⇌), indicates a reversible reaction.
- The resonance arrow (↔), this double-headed arrow indicates the resonance structures of two or more species. There is a difference in the arrangement of their electrons and nothing else. It is important to observe that the molecule does not shuffle back and forth between these forms but rather they give a true picture that the molecule is a combination or hybrid of these structures.
- The broken arrow is used to indicate reactions that don’t work i.e. in nucleophilic substitutions fluorine is considered as a bad leaving group and quinine cannot be synthesized from the oxidation of aniline.
- The Retrosynthesis arrow is meant to illustrate the process of breaking down a complex molecule into simpler starting materials. This is considered as a useful planning device to highlight a key strategy used for a molecule built up.
- Curly arrows are used to show the movement of both the electron pairs and single electrons during organic reaction mechanisms. One can’t use curly arrows for any other purpose.
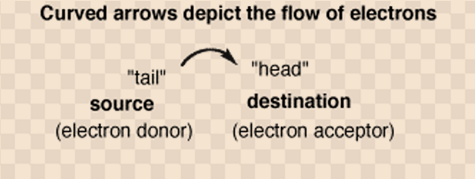
Curly arrows showing the movement of electron pair
- Symbolic representation
- Explanation
The tail of an arrow indicates where the electron pair starts. One must show the electron pair either as a bond or if it is alone pair than you have to represent it as a pair of dots. Don’t forget that a lone pair indicate a pair of electrons at the bonding level which is not currently being used to join on to anything else.
The head of an arrow is where you want the electron pair to end up.
- Example
Let’s take an example of ethene and hydrogen bromide. Here one of the two bonds between the two carbon atoms breaks. Simply, that bond is a pair of electrons. These electrons move towards hydrogen of the HBr to form a new bond. At once the pair of electrons in the hydrogen-bromine bond moves down on to the bromine atom.
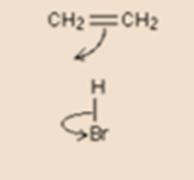
It is not necessary to draw the pairs of electrons in the bonds as two dots. It’s enough to draw a line to represent bond but you can put two dots too if you are willing.
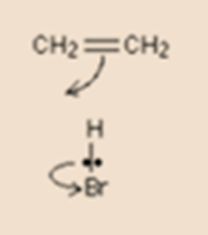
Note that the arrowhead points between the Carbon and hydrogen because that’s where the electron pair ends up. Also, notice that the curly arrow is used to represent the electron movement between H and Br even though the electron pair moves straight down. We have to indicate electron pair movement as curly arrows and not as a straight one.
The second step of this reaction explains how we can use a curly arrow if a lone pair of an electron is involved.
The first step leaves us with a positive charge on the right-hand carbon atom and a negative bromide ion. we can think of the electron shown on the bromide ion as being the one which originally made up the hydrogen-bromine bond.
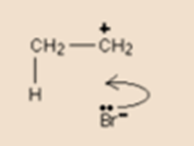
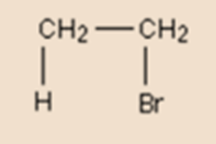
Bromide ion having a lone pair on it moves to form a new bond between bromine and the right-hand carbon atom. This movement is again shown by a curly arrow. Observe again, that the curly arrow points between the carbon and the bromine because of it a point where the electron pair end up.
This gives us a product of this reaction named bromoethane
Curly arrows showing the movement of a single electron
- Symbolic representation
the common use of the curly arrows is to indicate the movement of pairs of electrons but we can use the same arrows to indicate the movement of a single electron. But to indicate a single electron movement we have put a single line at the arrowhead rather than two lines.

- Example:
Let’s take an example of the polymerization of ethene, the first step can be shown as follows

We can draw the dots showing the concerning electrons. The half arrows indicate where electrons will go.
Curly Arrows Mechanism (Golden Rules)
- Curly arrows are used to indicate the movement of pairs of electrons
- Curly arrows always flow from an electron-rich species to an electron-poor species. i.e. from the nucleophile to electrophile.
- They get a start from lone pairs or bonds and end up either between a pair of atoms on an atom.
- There should be a balance in charges in any particular step.
- If you take out an electron from a bond, that bond will be broken.
- If you place electrons between two atoms, a bond is formed.
Consider a simple example
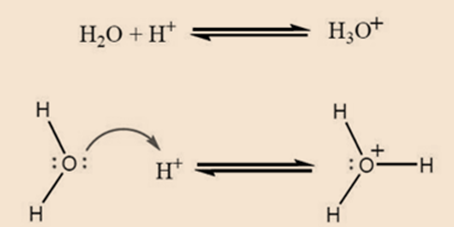
The curly arrow in an above reaction starts from a lone pair of the oxygen (a nucleophile) and ends on the proton (an electrophile). This shows that it flows from an electron-rich species to an electron-poor species. Charges are in balance as there is a positive charge on both sides of the equilibrium arrows. As the electrons are placed between two atoms (the oxygen and the proton) a bond is created.
References
http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch01/ch1-6-1.html
http://www.ch.ic.ac.uk/local/organic/tutorial/arrow_pushing.pdf
https://www.dur.ac.uk/chemistry/outreach/dusting/students/arrows/
https://www.chemguide.co.uk/basicorg/conventions/curlies.html?fbclid=IwAR2cyDNTZ-pXZnNU8uBAwl6GBLC37FYVskCj9RqfkyrskX8qMMNQOtbtxko
https://www.masterorganicchemistry.com/2011/02/09/the-8-types-of-arrows-in-organic-chemistry-explained/
