Introduction to carbonyl group
In organic chemistry, carbonyl group is a functional group where carbon and oxygen atom are connected with a double bond. The compounds contain carbonyl group are known as carbonyl compounds. Carbonyl group refers to a carbon monoxide can also attached to an inorganic atom as a ligand that is called metal carbonyl, e.g. nickel carbonyl.
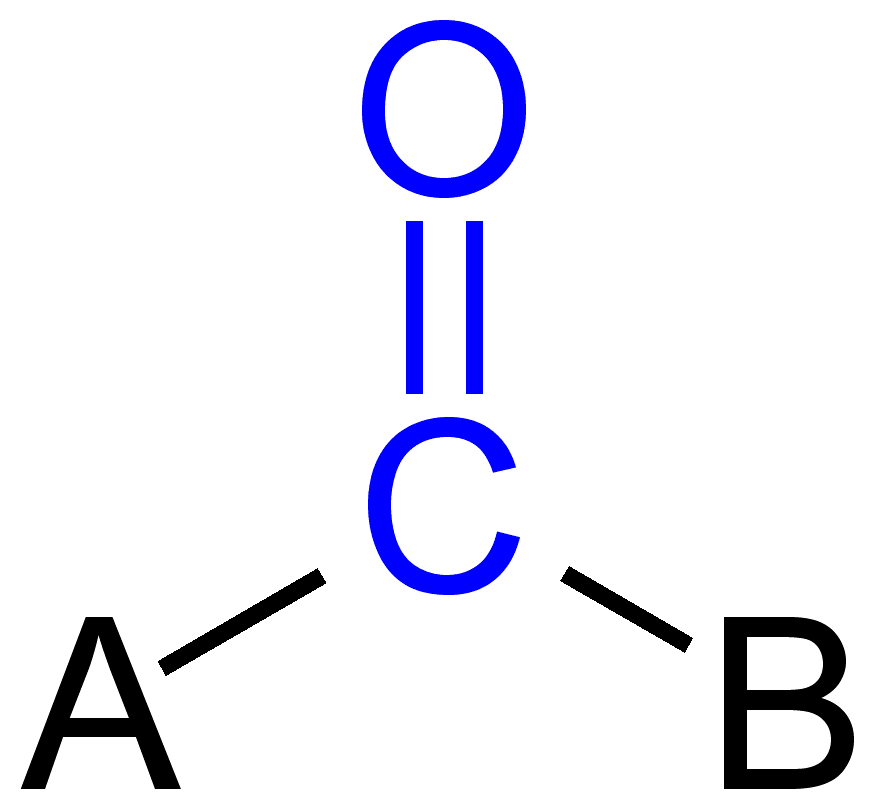
Some carbonyl groups
Aldehydes (RCHO), ketones (RCOR’), carboxylic acid (RCOOH), carboxylate ester (RCOOR’), amide (RCONR’R”), acid halide (RCOX), acid anhydride (RCO-O-OCR’) and so on.
Double bond in carbonyl group
The carbon and oxygen atom in carbonyl group are normally sp2 hybridized and thus plannar. Because of the presence of oxygen as an electronegative atom, the double bond in carbonyl group is different than the double bond in alkenes in terms of reactivity. Because of more electronegativity and presence of two lone pair of electrons, oxygen becomes partially negative charge while carbonyl carbon generates partially positive charge and thus polarity is observed.
Carbonyl resonance structure
Because of the presence of oxygen as an electronegative atom, carbonyl group shows resonance structure. Resonance structure of carbonyl group affect the reactivity of the compound. The more electronegative oxygen atom draws away electron from carbon center makes the carbonyl carbon partially electropositive and the oxygen partially electronegative. Thus polarity generates within the carbonyl compound.
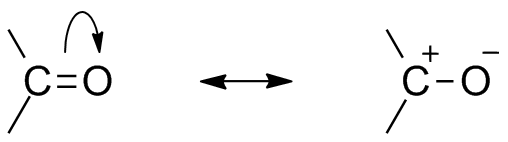
This electropositive carbonyl carbon or electrophile can now be attacked by a nucleophile.
Reactivity
Carbonyl Alkylation
Alkylation of carbonyl group by nucleophilic addition reaction using Grignard reagent are shown as follows:

Physical properties of carbonyl compounds
- The boiling point of aldehydes and ketones are higher than the boiling point of ethers and alkanes of similar molecular mass.
- Again the boiling point of aldehydes and ketones are lower than the corresponding alcohols.
- Many higher aldehydes have pleasant odor and used in perfumes or as an artificial flavoring whereas the lower member of their homologous series have pungent odor.
- Higher ketones have bland odor and lower have pleasant odor, such as acetone.
- Small aldehydes and ketones are soluble in water but as the chain length increase the solubility decreases.
- The van der Waals dispersion forces get stronger as the molecules get longer.
H-bonding in carbonyl goup
Because of the formation of H-bonding between the carbonyl oxygen and the hydrogen of water molecule, the lower carbonyl compounds are soluble in water. But as the chain increases, it comes on the way to form hydrogen bond and the solubility decreases.
Van der Waals dipole-dipole attraction
Because of the polarity in carbonyl group and the presence of dispersion forces there will be an attraction between two nearby molecules. This causes the boiling point of carbonyl compounds go higher that the corresponding alkanes. In following table notice that the boiling point of carbonyl compounds are higher than the corresponding alkanes and lower than the alcohols.
| Molecule | Group | Boiling point (o C) |
| CH3CH2CH3 | Alkane | -42 |
| CH3CHO | Aldehyde | +21 |
| CH3CH2OH | Alcohol | +78 |
Spectroscopy
Infrared spectroscopy
The absorption of carbonyl group depends on the geometry of the molecule. Usually the absorption of infra light shows at at wavenumbers approximately 1600–1900 cm−1 (5263 nm to 6250 nm).
Nuclear Magnetic Resonance
The carbon-NMR of carbonyl carbon shows peak approximately between 160-220 ppm.
