Definition of alkenes
Alkenes are hydrocarbons that contain carbon carbon double bond (C=C). The general formula of alkenes are CnH2n in comparison to alkanes with general formula CnH2n+2. Alkanes are saturated with hydrogens, while alkenes are two hydrogen less than alkanes. Thus it is known as unsaturated hydrocarbons. For example:
![Alkene 13 By Meir138 (Own work) [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/0/0b/Alkene.png)
Nomenclature
IUPAC system
In IUPAC system the alkenes are named by changing the suffix of alkanes –ane to –ene. The steps to write the name of the alkenes are:
Step 1: The name of alkenes are started with the name of the longest chain contains the double bond.
Step 2: The numbering of the carbon started from the nearest end carbon of the double bond.
Step 3: The position of the double bond is mentioned by a number before the name of the longest chain hydrocarbon.
Step 3: In case of sustituents the position and name of substituent will then be prefixed to it.
For example:
Common system
In common system the alkenes are named by changing the suffix of alkanes from –ane to –ylene. The nearest end carbon of the double bond in the longest chain is designated as greek letter α, then the next one β and so on.
Structure
The carbon atoms that create double bond, are sp2 hybridized. In a double bond, the two carbons are bonded together by overlapping two sp2 hybrid orbitals head to head, forming a sigma (σ) bond. The other two sp2 orbitals are overlapped with the s orbitals of two hydrogen atoms. The two unhybridized p orbitals of two carbon atoms are overlapped side to side to form π bond. Because of the presence of elctron rich π bond alkenes are more reactive than alkanes.
Isomerism
Depending on the position of the double bond, position isomers are possible. For example:
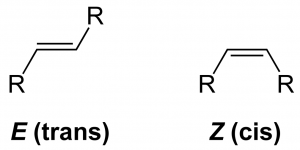
The p orbitals of two carbons overlapped and form a electron cloud above and below the single bond. Thus the rotation around this sigma bond is restricted. Due to this restricted rotation around the double bond, geometrical isomerism is possible in alkenes. For the alkene with general formula RCH=CHR, if the two hydrogens stays in the same side is called cis-isomer and if they are in opposite side is called trans-isomer. For example:
Physical properties
- Lower alkenes are gases in room temperature. As the carbon chain increases the alkenes are liquid and the longer chain alkenes are solid in room temperature. Most of the alkenes are colourless and odourless.
- As alkenes have no polarity. It sparingly dissolved in water and readily dissolved in organic solvent.
- The IR spectrum shows C-H stretching at 3000-3100 cm-1.and C=C stretching at 1620-1680 cm-1.
Preparation of alkenes
Elimination reaction
One of the common synthesis of alkene is by the elimination reaction from alkyl halide, alcohol and similar compound. Alkyl halide undergoes dehydrohalogenation and alcohol undergoes dehydration to form corresponding alcohol. For example: in basic medium alkanes produces alkenes with the elimination of hydrochloric acid.
![Alkene 16 By Rifleman 82 (Own work) [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/2/26/Alkene_Formation_V.1.png)
Reactions
Alkenes perform many addition reactions via the mechanism of electrophillic addition.
Hydrohalogenation
Hydrohalogenation is the the addition of hydrogen halide to form alkyl halide.
![Alkene 17 By Anonymouse197 (Own work) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/3/39/Alkene_and_Hydroge_Halide.png)
Halogenation
Halogenation is another addition reaction where halogen is added to form dihaloalkane.
![Alkene 18 By Benjah-bmm27 (Own work) [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/3/33/Alkene-bromine-addition-2D-skeletal.png)
Summary
- Alkenes are hydrocarbons that contain carbon carbon double bond (C=C).
- In IUPAC system the alkenes are named by changing the suffix of alkanes –ane to –ene.
- In common system the alkenes are named by changing the suffix of alkanes from –ane to –ylene.
- The carbon atoms that create double bond, are sp2 hybridized.
- Due to the restricted rotation around the double bond, geometrical isomerism is possible in alkenes.
- It sparingly dissolved in water and readily dissolved in organic solvent.
- One of the common synthesis of alkene is by the elimination reaction from alkyl halide, alcohol and similar compound.
- Alkenes perform many addition reactions via the mechanism of electrophillic addition.

![Alkene 14 By Rifleman 82 (Own work) [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/b/bb/Alkene_nomenclature.png)