Definition
Hydrogen bonding is a weak type of force which forms a dipole-dipole interaction between two molecules within the same molecule. Depending upon different contexts, its definition has been changing. According to earlier definitions “Hydrogen bonds is an interaction between the covalent pair A—H (donor) to a nearby electronegative atom B or X (acceptor). And “A” is more electronegative [1] [2].
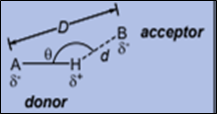
Fig 1: Basic Structure of intermolecular hydrogen bonding.
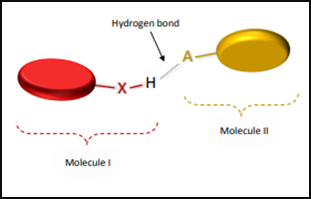
Fig 2: Hydrogen bond donor and hydrogen bond acceptor molecule.
With the context of van der wals interactions “Hydrogen bond exists between the functional group A-H and atom or group of atoms X in same or different molecules when below mentioned conditions are fulfilled i.e.
a) Evidence of bond formation.
b) Hydrogen already making a bond to some atom and this linkage will be an additional one [3].
Primary Features of hydrogen bonding
Due to the following main reasons, hydrogen bonding is originated between molecules. Firstly, Hydrogen is attached to one of most electronegative elements and this bonding causes hydrogen to acquire a positive charge. Secondly, all atoms, to which hydrogen is attached, are not only negative but that each element should have one active lone pair present in the outermost shell.
Types of hydrogen bonds
The Following two types of hydrogen bonds exist depending upon the position of elements that are bonding together by these bonds.
- Intramolecular hydrogen bonding:
Intramolecular hydrogen bonds are those which occur within one molecule. This is mainly due to the presence of two functional groups of a molecule that are capable of forming hydrogen bonds with each other. For this to happen, both a hydrogen donor an acceptor must be present within one molecule, and they must be within proximity of each other in the molecule. For example, Intramolecular hydrogen bonding occurs in ethylene glycol between its two hydroxyl groups and nitrophenol.
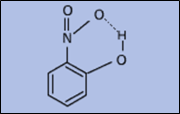
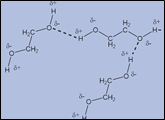
Figure 3: Examples of Intramolecular hydrogen bonding in Ethylene glycol (left) and O-nitro phenol (Right)
- Intermolecular hydrogen bonding:
Hydrogen bonds can also occur between separate molecules of the same substance. They can occur between any numbers of molecules as long as hydrogen donors and acceptors are present in positions in which they can interact. For example, intermolecular hydrogen bonds can occur between NH3 molecules, between H2O molecules alone, or between NH3NH3 and H2OH2O molecules. HFHF is also another example of intermolecular hydrogen bonding. And this bonding gives a unique set of physical properties to these molecules in bonded form [4].
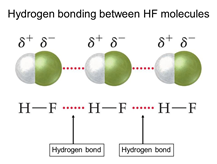
Figure 4: Intermolecular hydrogen bonding: (a) NH3-H2O (b) H2O- H2O (c) H2O –NH3 (d) HFHF
Hydrogen Bonding in HF
One important example of intermolecular hydrogen bonding is among HF molecules. Hydrogen bonding, in this case, is originated in the following way:
Hydrogen contains one electron, and fluorine requires one electron to become stable, so the bond forms readily when the two elements interact. As a result of this interaction; hydrogen fluoride is formed. These HF molecules further make chains with each other through hydrogen bonding interactions. When two hydrogen fluoride molecules interact with each other then, they form a zig-zag structure involving interaction between positively charged hydrogen of one molecule with negatively charged fluoride of another molecule [5].
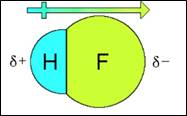
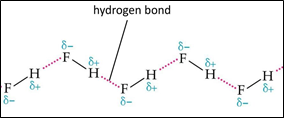
Fig 3: Linear structure showing hydrogen bonding between HF molecules and sigma positive and sigma negative charges,
Physical Properties of hydrogen fluoride
Hydrogen fluoride is a colorless gas that is corrosive in nature. When hydrogen fluoride is dissolved in water, hydrofluoric acid is formed. HF forms orthorhombic crystals below −83.6 °C (−118.5 °F), consisting of zig-zag chains of HF molecules. The HF molecules, with a 95 pm length H–F bond, are linked to nearby molecules by intermolecular H–F Hydrogen
bonding having a distance of 155 pm. Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting of an average of only five or six molecules [6].
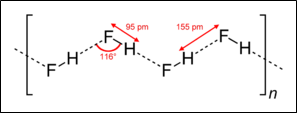
Figure 5: Bond length and Bond angle
Bonding angle
The bonding angle of HF hydrogen bonding is 115 degrees. This gives it an orthorhombic structure, as this angle is purely dependent on outermost orbitals.
Strength of HF molecule:
Hydrogen fluoride, HF, is the only halide that can form hydrogen bonds. Since fluorine is the most electronegative element, the difference in electronegativity between itself and hydrogen will be the biggest of the group. However, in the case of the other halides, the inability to form hydrogen bonds has another important reason behind it.
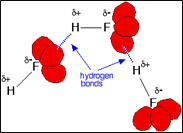
Fig 6: The presence of charge at molecules ends are well explained by orbitals in red color.
Two reasons are the atomic size and electronegativity difference.
- Electronegativity difference:
Bigger the electronegativity difference between hydrogen and the halide it’s bonded to, the greater the partial positive charges on the hydrogen atom. Less electronegative halides imply a smaller difference in electronegativity with hydrogen. This will result in the formation of a smaller partial positive charge on the hydrogen atom.
- Atomic size:
The Importance of atomic size is discussed here: The smaller the atomic size of the halide, the more negative its lone pairs of electrons will be. As we go down to group 17, the lone pairs will occupy increasingly bigger orbitals due to the increased energy levels on which they are added. This will allow negative charge to spread on greater and thus less concentrated. So, two factors go together here i.e. one molecule’s hydrogen and another molecule’s lone pairs so it may lead to the formation of no hydrogen bonds [6].
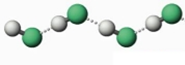
Fig 7: Hydrogen bonds in hydrogen fluoride, Hydrogen atoms are denoted in white and Fluorine atoms in green
- Effect on Boiling point:
The higher boiling point of HF relative to other halides, such as HCl, is due to hydrogen bonding between HF molecules, as indicated by the existence of chains even in the liquid state. Although they belong to the same group in the periodic table, they are heavier and having less electronegative than fluorine. This is the reason of HF being liquid as room temperature and other halides are gaseous [7].
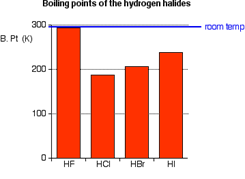
Figure 8: Graph comparing boiling points of halides.
- Chains of hydrogen fluoride:
Although it is a diatomic molecule still, it forms relatively strong intermolecular hydrogen bonds. Solid HF consists of zig-zag chains of HF molecules [8].
- Low acidic strength:
Hydrogen Bonding in HF is the reason for its low acidic strength. The partially positive hydrogen is trapped between two highly electronegative atoms of Fluorine. So; it becomes difficult for HF to release a proton. That’s why its acidic strength is low as compared to other halides. An aqueous solution of HF is called Hydrofluoric acid. Dilute hydrofluoric acid is a weak acid and the concentrated HF is strong acid due to the formation of hydrogen-bonded ion pairs [9].
- Comparison with other hydrogen halides:
Hydrogen fluoride boils at 20 °C in contrast to other halides, which boil between −85 °C (−120 °F) and −35 °C (−30 °F).
- Effect on viscosity:
Substances having a hydrogen bonding in it have a usually higher viscosity than those which don’t have any hydrogen bonding in them. Substances that have the possibility for multiple hydrogen bonds exhibit even higher viscosities. The same is the case of HF. The hydrogen bonding between HF molecules gives rise to high viscosity in the liquid phase and lowers than expected pressure in the gas phase.
- High Hydration Enthalpy:
If we look at energetic of HF hydrogen bonding, then we will understand the fact that we have to put a lot of energy to break the HF bond. And in the same way when Fluoride ions are surrounded by water molecules, then a lot of energy is released as well. So, high hydration enthalpy of fluoride ions somewhat compensates for high HF bond strength. The presence of hydrogen bonding in the HF molecule is making it unique concerning physical and chemical properties such as boiling point, viscosity, and acid strength.
References
- Jeffrey, G.A. and Saenger, W. (1991) Hydrogen Bonding in Biological Structures. Springer-Verlag, Berlin. https://doi.org/10.1007/978-3-642-85135-3
- Kojić-Prodić, Biserka & Molcanov, Kresimir. (2008). “The Nature of Hydrogen Bond: New insights into Old Theories”. Acta Chimica Slovenica. 55. 692-708.
- http://evans.rc.fas.harvard.edu/pdf/smnr_2009_Kwan_Eugene.pdf
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Hydrogen_Bonding
- Nmentel G C & McClellan A L. The hydrogen bond. San Francisco: W.H. Freeman, 1960. 475 p. [University of California, Berkeley, and California Research Corporation, Richmond, CA]
- McLain, S. E., Benmore, C. J., Siewenie, J. E., Urquidi, J. and Turner, J. F. (2004), On the Structure of Liquid Hydrogen Fluoride. Angewandte Chemie International Edition, 43: 1952-1955. doi:10.1002/anie.200353289
- https://socratic.org/questions/556e7edf581e2a437c258042
- http://www.whatischemistry.unina.it/en/hbond.html)
- https://www.chemguide.co.uk/inorganic/group7/acidityhx.html
Figures
- http://evans.rc.fas.harvard.edu/pdf/smnr_2009_Kwan_Eugene.pdf
- https://www.ccdc.cam.ac.uk/Community/educationalresources/teaching-modules/Teaching%20Tutorial%20-%20Hydrogen%20Bond.pdf
- http://www.chm.bris.ac.uk/motm/ethylene-glycol/glycoljs.htm
- https://chemistry.stackexchange.com/questions/60769/why-o-nitrophenol-is-more-volatile-than-p-nitrophenol
- https://www.chemguide.co.uk/inorganic/group7/acidityhx.html
