Hydroxide Definition
Hydroxide is a negatively charged ion comprised of oxygen and hydrogen atom bonded covalently with each other. It normally attached with a positively charged metal to form a base. e.g. NaOH. In aqueous solution, these base give hydroxide ion OH–. According to Arrhenius substance that forms hydroxide ion in aqueous solution is base.
![Hydroxide 21 By Jynto (more from this user) [CC0], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/6/6d/Hydroxide_anion_or_hydroxyl_radical_spacefill.png)
Structure of hydroxide ion
The electronic configuration of oxygen and hydrogen are 1S22S22P4 and 1S1 respectively.
In hydroxide ion, hydrogen and oxygen atom shared their two valence electrons to form a covalent bond. The extra electron of hydroxide ion comes from a metal atom when it was a part of a compound.
The metal atom has low ionization energy and gives electron to the oxygen of hydroxide to make ionic bond. e.g NaOH. In aqueous solution sodium hydroxide gives Na+ and OH– ions.
Hydroxides in different types of compounds
Organic hydroxides
Negatively charged hydroxide ion can attach with a positively charged organic ion. As for example:
![Hydroxide 24 By Dschanz (own work (drawn with MDL ISISDraw®)) [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/3/3d/Choline_hydroxide.png)
Inorganic hydroxides
Hydroxide ions form ionic bond with different types of metals in periodic table.
With alkali metals
Most popular and most useful bases are formed with alkali metals, such as sodium hydroxide (NaOH), potassium hydroxide (KOH), lithium hydroxide (LiOH) etc. These are all strong bases.
With alkali earth metals
The beryllium hydroxide Be(OH)2 is amphoteric in nature. While the other hydroxides of this group like magnesium hydroxide Mg(OH)2, calcium hydroxide Ca(OH)2 etc. are strong bases and are soluble in water.
With transition metals
Transition metals form very unstable hydroxides using their +1 oxidation state. Such as silver hydroxide Ag(OH) is unstable and it readily decomposes to form Ag2O. But M(OH)2 and M(OH)3 are stable and can be made by increasing the pH of the aqueous solution.
With other elements
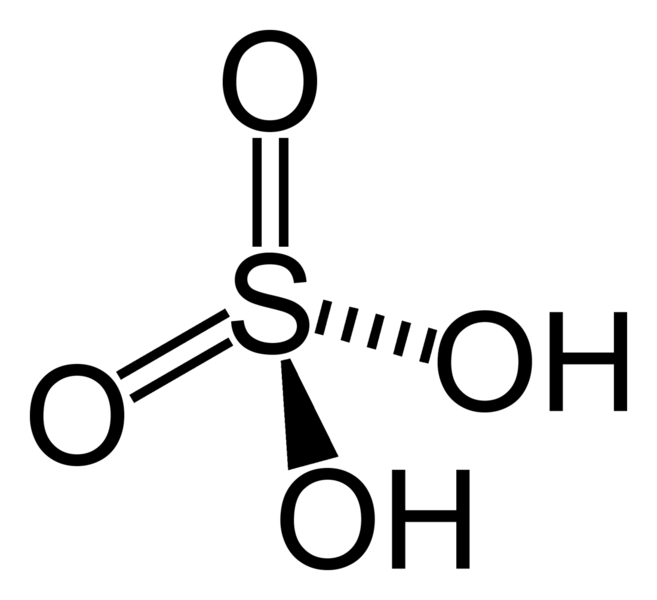
Hydroxides can attach with other elements of the periodic table. It is not necessary that because of the presence of hydroxide ions, the compounds will be basic in nature. It can be acidic or it can show any other properties. As for example: H2SO4, H3PO4 both are acidic in nature.
Functions of hydroxide
Hydroxide ions can function as base, ligand, nucleophile or a catalyst.
Hydroxide ions as a base
When hydroxide ions attached with a metal, it act as a base. The hydroxide ion containing bases have following properties:
![Hydroxide 27 By Matthew Sergei Perrin from Auckland, New Zealand (Sodium Hydroxide 6mol Corrosive Lab Chemicals) [CC BY 2.0 (http://creativecommons.org/licenses/by/2.0)], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/f/f0/Sodium_hydroxide_solution.jpg)
![Hydroxide 28 By Blazius (Own work) [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/0/09/Sodium_hydroxide_burn.png)
- have high pH (7-14)
- can make foam in aqueous solution
- slippery in aqueous solution
- reacts with acid
- toxic
- corrosive and
- can be very dangerous.
Arrhenius acid and base reacts to form salt and water. For example, sodium hydroxide and hydrochloric acid reacts to form sodium chloride salt and water.
NaOH + HCl → NaCl + H2O
Here negatively charged hydroxide ion reacts with positively charged hydrogen ion to form water.
OH– + H+ → H2O
Hydroxide ions as a ligand
Ligands are electron pair donar or a lewis base which donates electrons to a central metal atom, known as electron pair acceptor or lewis acid, to form a neutral molecule or ion. Hydroxide ions can donate electron pair from its outer most shell thus can act as a ligand or a lewis base. As for example: aluminate ion [Al(OH)4]–.
Hydroxide ions as a nucleophile
Hydroxide ions act as a nucleophile in different types of reactions like- nucleophilic acyl substitution, nucleophillic aliphatic substitution, nucleophilic aromatic substitution, elimination reaction and so on. Among these nucleopphilic acyl substitution is described here.
In nucleophilic acyl substitution, as a nucleophile, hydroxide ion attacks to the carbonyl centre to form an alcohol and acid.
![Hydroxide 30 By Ckalnmals (Own work) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/1/1d/Nucleophilic_Acyl_Substitution_with_a_Labeled_Oxygen.png)
Hydroxide ions as a catalyst
Hydroxide ions can act as a catalyst in different types of reactions. Among these degradation of hemiacetal via E1cB reaction mechanism is explained. In this type of reaction, hydroxide ions (OH–) of a base takes away proton (H+) leaving the negatively charged oxygen which eventually form ketone and alcohol.
![Hydroxide 31 Physchem 13 at English Wikipedia [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/b/b3/An_example_of_the_E1cB_reaction_mechanism_in_the_degradation_of_a_hemiacetal.jpg)
