Le Chatelier’s Principle Definition
Le Chatlier’s principle is also known as “Chatelier’s principle” or “The Equilibrium Law”. Le Chatelier’s principle states that if a dynamic equilibrium is disturbed by changing the conditions (such as concentration, temperature and pressure changes) , the position of equilibrium shifts to counteract the change to reestablish an equilibrium. If a chemical reaction is at equilibrium and experiences a change in pressure, temperature, or concentration of products or reactants, the equilibrium shifts in the opposite direction to adjust itself to reduce the change.
Here we will discuss about the effects of these three changes what causes the equilibrium to shifts to minimize the stress.
- Effect of a change in concentration
- Effect of a change in pressure
- Effect of change in temperature
1. Effect of a change in concentration
According to Le Chatelier, when concentration of any of the reactants or products is changed, the equilibrium shifts in such a direction that can reduce the change of concentration.
Increasing a concentration
Let us consider a equilibrium reaction of A and B to form a product C.

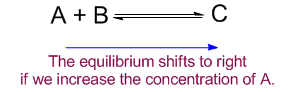
Here if we increase the concentration of A by adding more of that the equilibrium will shift from left to right by reacting with B to reestablish the equilibrium. And the equilibrium will shift from right to left in case the concentration of the product C is increased in the system.
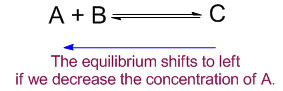
Decreasing a concentration
In opposite way if the concentration of A is decreased the equilibrium will shift from right to left to minimize that effect. And the equilibrium will shift from left to right if the concentration of C is decreased.
2. Effect of a change in pressure
This applies only to the equilibrium reactions where 1 or more reactants or products are in gas phase. According to the principal, the change of pressure shifts the equilibrium in such a direction to reduce the change.
Increasing the pressure
When the pressure is increased in any equilibrium reaction where 2 gas molecules of A produce 1 gas molecule of B, the equilibrium will shift from left to right.
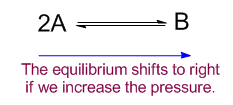
As total pressure is the pressure of the total no. of molecules present in the system, so if the number of molecules increases the pressure will be increased. Thus to minimize the pressure applied on the system, equilibrium will shift in such a direction to produce fewer molecules. For this case it will shift to right to form more product.
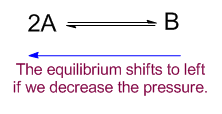
Decreasing the pressure
When the pressure is decreased the equilibrium will shift to produce more gaseous molecules. In this case it will shift from right to left.
3. Effect of change in temperature
Equilibrium reactions consists of two opposing reactions. If the forward reaction proceeds by the evolution of heat (exothermic reaction), the reverse reaction must happens by absorption of heat (endothermic reaction). For this we must need to know whether heat is absorbed or evolved during the reaction. According to the law the equilibrium will shift in such a way that can counteract the change.
Increasing the temperature
Let us consider an exothermic reaction where, if heat is evolved in forward reaction, the same amount of heat is absorbed in backward reaction (endothermic reaction). Suppose the reaction is in equilibrium at 3000 C.
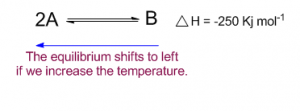
Thus increasing the temperature to 5000 C favors the backward reaction as backward reaction occurs by the absorption of heat from surroundings (endothermic reaction).
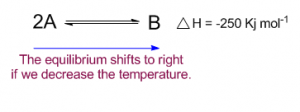
Decreasing the temperature
If we decrease the temperature to 2000 C the equilibrium will shift to right (forward reaction) to produce more product. So more heat is evolved as this is an exothermic reaction to achieve the equilibrium temperature 3000 C.
Summary
- Increasing the concentration of reactants, the equilibrium shifts to right.
- Increasing the concentration of products, the equilibrium shifts to left.
- Decreasing the concentration of reactants, the equilibrium shifts to left.
- Decreasing the concentration of products, the equilibrium shifts to right.
- Increasing the pressure, the equilibrium shifts to form fewer gas molecules.
- Decreasing the pressure, the equilibrium shifts to form gas molecules.
- No effect of pressure when the number of molecules are same on both sides.
- No effect of pressure when inert gas is added in any reaction.
- Increasing the temperature, the equilibrium favors the endothermic reaction.
- Decreasing the temperature, the equilibrium favors the exothermic reaction.
