Isomerism Definition
Compounds which have same molecular formula but differ in modes of combination or arrangement of atoms within the molecule are known as isomers and this phenomenon is know as isomerism. Isomers can have different physical or chemical properties.
As for example, the cis and trans isomers of but-2-ene are as follows:
Classification of isomerism
There are two main types of isomerism- structural isomerism and stereoisomerism. These can be further classified as,
Structural Isomerism
Structural isomerism, or constitutional isomerism, is a type of isomerism where isomers have same molecular formula but have different arrangements of atoms within the molecule.
Structural isomerism can be further classified as:
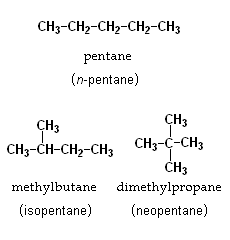
1. Chain isomerism
Chain isomerism is a type of structural isomerism where the isomers have same molecular formula but they differ in the order in which the carbon atoms are bonded to each other.
2. Position isomerism
Position isomerism is a type of structural isomerism where the main carbon skeleton are same but they differ in the position of functional group attached to it.
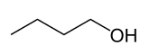
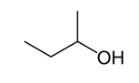
Butan-1-ol Butan-2-ol
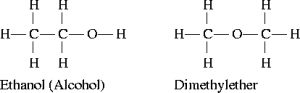
3. Functional group isomerism
Functional group isomerism is a type of structural isomerism where isomers have same molecular formula but differ in functional group.
4. Metamerism
Metamerism is a type of structural isomerism where the isomers have same molecular formula but differ due to the different number of carbon atoms or alkyl groups on either side of functional group ( i.e., -O-,-S-, -NH-, -C(=O)-).
![]()
Diethyl ether methyl propyl ether
5. Tautomerism
Tautomerism is a special type of structural isomerism where the isomers stays in dynamic equilibrium with each other by simple proton transfer in an intramolecular fashion.
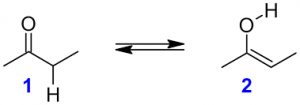
2-butanone 2-butanol
Keto-form Enol-form
Stereoisomerism
Stereoisomers are isomers that have same molecular formula, same sequence of bonding of atoms but differ in their three dimensional orientation of atoms in space. Stereoisomerism are of two types:
1. Diastereomerism
Diastereomers are a type of stereoisomers where two isomers differ in there configuration and are not mirror image of each other. Two types of diastereomers are possible:
a) Cis-trans isomerism
Geometrical isomerism or cis-trans isomerism arises because of the restricted rotation around the carbon-carbon double bond.
b) Conformational isomerism
Conformational isomerism is a diastereomerism where two isomers differ because of the rotation around the carbon-carbon single bond.

2. Enatiomerism
Isomers which are non-superimposable mirror images of each other are known as enantiomers.

These compounds are optically active and can rotate the plane polarized light. Thus it is also known as optical isomerism. The isomer which can rotate the plane polarized light from left to right (or clockwise) is known as dextrorotatory and which can rotate from right to left (or anticlockwise) is known as laevorotatory. A equal mixture of two enantiomer is known as racemic mixture. Such a mixture is optically inactive as they rotate the plane polarized light in opposite direction and thus cancel each other.
Isomerization
Isomerization is the conversion of one isomer into another. This is also known as rearrangement reaction. The isomers have normally similar bond energy and thus normally exist together in different ratio. So it is possible to convert them easily by crossing the energy barrier between them.
