Introduction
It is also known as the Haber – Bosch process or Synthetic Ammonia process. Haber’s process is considered as one the most beneficial and efficient industrial processes to be used for the production of ammonia which is a colorless gas having a distinct odor. In the 20th century Fritz Haber, a German chemist developed a high-pressure devices and appropriate catalysts to carry out the process on a laboratory scale. He received the Nobel prize for his great achievement of manufacturing ammonia economically feasible.
Before this in 1910 another chemist Carl Bosch created a design and a machine for the production on a large industrial level. It was, however, an important development in this field. He was hired by a German company BASF which assigned him the task of manufacturing the ammonia on an industrial level.
Before the development of the Haber process, it was very difficult to produce ammonia on an industrial scale with early methods i.e. Birkeland-Eyde process and Frank-Caro process both being highly inefficient.
Ammonia (NH3) is considered as one of the very essential hydride that is present in an atmosphere in trace amount. An atmospheric ammonia is formed by the bacterial decomposition of nitrogenous matter of the plants and animals.
In 1774 ammonia was isolated by the reaction of ammonium chloride and lime. It was known as alkaline air. In 1788, Berthelot indicated that ammonia is a compound of hydrogen and nitrogen. Later in 1800, Davy established its formula NH3.
Definition:
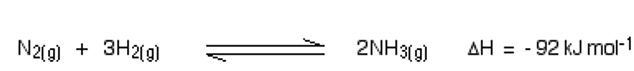
Ammonia (NH3) is manufactured during Haber’s process. During this process, a pure Nitrogen and Hydrogen gases are reacted in the ratio of 1:3 by volume at high temperatures (400 C – 500 oC) and pressure of 150-200 atm in the presence of a catalyst, iron, or Molybdenum as a promoter. This is a reversible reaction, and ammonia production is exothermic, with Fe/Mo as catalyst and atmospheric pressure of 200-900 atm.
Raw materials:
- Nitrogen: It is a very unreactive gas because the molecules are held together by a strong triple bond. It is extracted from the distillation of liquid air. It involves methane and removal of oxygen.
- Hydrogen: It is produced industrially by reacting methane with steam in the presence of nickel catalyst at a temperature of about 750 C , separating the carbon and hydrogen atoms in the natural gas.
CH4(g) + H2O → H2(g) + CO(g)
Carbon monoxide must be removed before feeding into Haber process. Hydrogen is also produced from biomass and water using electrolysis process.
Process Details
According to this diagram, nitrogen gas is taken from the air. Then it is combined with hydrogen atom that is extracted from natural gas in the ratio of 1:3 by volume i.e. in this process the mixture of nitrogen and hydrogen is added in the reactor by the ratio of 1:3 means 1volume of nitrogen is going to be added with 3volumes of hydrogen. According to Avogadro’s Law during same temperature and pressure, an equal number of gases contains an equal number of molecules.
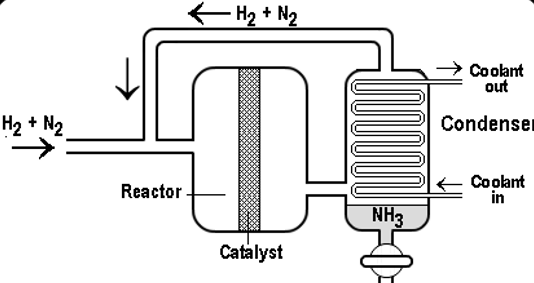
These gases are then allowed to pass through the beds of catalyst where cooling takes place. This is required to maintain equilibrium constant. In each pass different forms of conversion takes place and unreacted gases are recycled. Usually, iron is used as a catalyst while a temperature of 400 -450 oC and a pressure of 150-200 atm is maintained. Approximately 15% of the nitrogen and hydrogen is converted into ammonia (this may vary from plant to plant) through continual recycling of unreacted gases like nitrogen and hydrogen, and we attain the results about 98%. Finally, the ammonia formed is cooled down to get a liquid solution which is then collected and is stored in containers.
Equilibrium Consideration on Haber’s process:
The Haber’s process involves an equilibrium reaction. Here we need to know about Le Chatelier’s principle because it tells us how reaction conditions will impact on the production of ammonia.
- If the pressure on the reaction system is increased, the equilibrium will move to the right as the number of moles of reactants is greater than that of products.
- If the temperature is lowered, the equilibrium will move to the right as the reaction system is exothermic.
So according to Le Chatelier’s principle, the most favorable conditions to produce ammonia on an industrial scale is high pressure and low pressure.
Uses of Ammonia:
Synthetic ammonia that is produced today by a reaction between hydrogen and nitrogen is the root from which all the nitrogen-containing products are derived. The worldwide ammonia production is greater than one thirty million tons and, this is the sixth-largest produced chemical. Ammonia is considered as the major raw material for agriculture and industry.
Production of ammonia through the Haber-Bosch process came in the early 1900s just before and during World War 1 and became a big wheel in the global sustainability very quickly. As Germany entered the war and its requirement became high to make nitrates for explosives and gunpowder. Other than the war, the animals waste was considered as the basic source of fertilizer which was not enough as the world’s agricultural requirement was increasing day by day. On the other side, global nitrate supplies came from limited composting of plants waste and from deposits of Chile that were mined by the British companies. As the war was going between Britain and Germany, the ammonia supply was greatly reduced. In short without development in science i.e. Haber process Germany may not be able to fight the war as long as it did. Some of its major uses are enlisted below:
- Fertilizer: worldwide more than 80% of the synthetic anhydrous ammonia is used as an agricultural fertilizer. An anhydrous ammonia is that type of ammonia which doesn’t contain water. The released nitrogen helps for plants i.e. crops to grow well by providing essential nutrients like zinc, selenium and boron.
- Cleaning agent: ammonium hydroxide which is also known as household ammonia is widely used in cleaning daily household products i.e. tubs, tiles, sinks, etc. it is also useful in cleaning greasy stains because ammonia quickly evaporates leaving glass neat.
- Refrigeration: it is also used as a refrigerating agent because it can absorb an appropriate amount of heat from is surroundings.
- Industrial uses: it is used to clean water supplies and manufacture of many products like explosives, pesticides, and dyes. It is also used as a stabilizer in paper and in rubber and beverage industries. It is also widely used in waste treatment and pharmaceuticals. It can be used in fabric industry such as nylon and other polyamides.
Economic and Environmental aspect
- Beforethe invention of Haber’s process another method known as Cyanamid process was used to manufacture ammonia, but this process consumed a large amount of electrical power and was far more labor intensive.
- Now the Haber’s process produces 450 million tons of nitrogen fertilizer per year. This is mostly anhydrous form of ammonia, ammonium nitrate and urea. The Haber’s process consumes about 3-5% of the world natural gas i.e. about 1-2% of worlds energy.
- Combining ammonia with herbicides and pesticides leads to an increase in the productivity of agricultural land:
- The crops that are harvested in the year 2000 would have required about four times more land to get average yield in year 1900 and the cultivated area would have required approximately half of the ice-free continents than 15% of the land that it is required today.
- The manufacturing of ammonia also participated in climatic change and many other environmental problems like leaching of nitrates into ground water, lakes, ponds and rivers; natural eco system is also affected from the deposition of nitrates and ammonia; eutrophication is also taking place as a result dead zones are expanding in coastal ocean waters; manufacturing of ammonia also causing greenhouse effect as it emits large amount of nitrous oxide (N2O) and becomes third important greenhouse gas followed by CO2 and CH4.
- The Haber-Bosch process is considered one of the largest contributors for building up of a reactive nitrogen in the biosphere which cause anthropogenic disturbance to the
- nitrogen cycle.
- The nitrogen use efficiency that is typically less than fifty percentage of the farm runoff from excess heavy use of fixed industrial nitrogen damaging biological habitats.
- Around 50% of nitrogen that is present in human tissues is originated from Haber-Bosch process.
Safety information:
Generally, no significant health effects can be found in humans that are exposed to natural environmental concentrations of ammonia but if the concentrations exceed than some of the instructions are as follows:
- While dealing with products containing ammonia, the area should be well ventilated.
- Wear proper clothing and eye protection.
- Extreme exposures to this chemical can irritate skin, eyes and lungs.
- Mixing of ammonia with chlorine bleach can be very toxic as it produces chloramines which is very toxic and can cause chest pain, shortness of breath, nausea, irritation to nose, eyes and throat and even fluid into the lungs.
- Accidental swallowing to this chemical can burn the mouth, throat, stomach and, can cause severe abdominal pain.
References:
https://byjus.com/chemistry/haber-process/
https://study.com/academy/lesson/the-haber-process-commercial-uses-chemistry.html
https://medium.com/@worldofchemical/manufacturing-of-ammonia-by-habers-process-d3a79f8ab52f
