Esterification Definition
Esterification is an equilibrium reaction to form ester mainly from alcohols and carboxylic acids. Esters can also be made from the reactions between acyl chlorides (acid chlorides) and alcohols, and from acid anhydrides and alcohols.
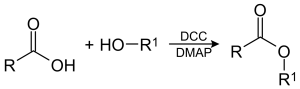
Here carboxylic acid and alcohol reacts to form an ester. Thus this is an esterification reaction. Esterification can occur in three different ways
- From carboxylic acid and alcohol
- From acid chloride and alcohol
- From acid anhydride and alcohol
1. From carboxylic acid and alcohol
Esters are mainly produced from carboxylic acid and alcohols by heating in presence of acid catalyst. Usually concentrated sulfuric acid is used as a catalyst. For example ethanoic acid and propanol reacts to form propyl-ethanoate and water.
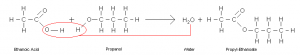
Preparation of ester
In a test tube
Carboxylic acids and alcohols are warmed together in presence of a few drops of concentrated sulphuric acid. The fruity smell proved the formation of esters. For a quick observation you can use small quantities of everything in a test tube and then heat it, in a hot water bath for a couple of minutes.
The smell is often distorted by the smell of carboxylic acid because of the reverse reaction to form carboxylic acid. By pouring the whole reaction mixture in a small beaker, the smell can be detected.
Small esters are fairly soluble in water but solubility decreases with the increase of chain length. Long chain esters form a thin layer on the surface. Acid and alcohol both dissolved in water and are tucked safely away under the ester layer. Longer chain esters smell, tend towards artificial fruit flavoring.
On a larger scale
To produce ester in large scale there are two different methods for short and long chain esters. Small esters are usually formed faster than bigger ones. For example, by heating a mixture of ethanoic acid and ethanol in presence of concentrated sulphuric acid, a small ester ethanoic acid can be made. Then the crude mixture is distilled off to get the ester in pure form.
Distilling the reaction mixture to collect esters, prevents the reverse reaction happening. Short chain esters do not have hydrogen bond, weakest intermolecular forces and thus lowest boiling point compared to the other elements in the reaction mixture. So by distillation we can easily separate ester from the reaction mixture. As this is an equilibrium reaction by removing the product from the reaction mixture, we can increase the production of ester.
Larger esters are slower than short chain esters. The reaction needs to be heated under the reflux for some time to produce an equilibrium mixture. The ester can be separated from the equilibrium reaction mixture by fractional distillation.
2. From acid chloride and alcohol
Esterification can also be done from acid chloride and alcohol at room temperature. After a vigorous (even violent) ester is formed with steamy acidic fume of hydrogen chloride. For example, benzoyl chloride and alcohol reacts to form ester.
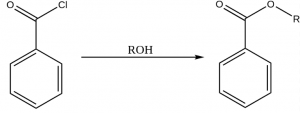
3. From acid anhydride and alcohol
The reaction between acid anhydride and alcohol is comparatively slower than with acid chloride and usually need to warm the mixture to get more esters. For example, 2,6-diiodophenol reacts with acid anhydride to form ester.
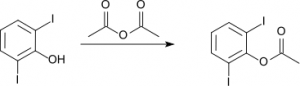
Summary
- Esterification is an equilibrium reaction to form ester mainly from alcohols and carboxylic acids. Esters can also be made from acyl chlorides (acid chlorides) and alcohols, or from acid anhydrides and alcohols.
- In small scale for a quick observation you can use small quantities of everything in a test tube and then heat it, in a hot water bath for a couple of minutes. By pouring the whole reaction mixture in a small beaker, the smell can be easily detected.
- On a large scale, heating a mixture of acid and alcohol in presence of concentrated sulphuric acid, a small ester ethanoic acid is made. Then the crude mixture is distilled off to get the ester in pure form.
- For large scale, to produce the longer chain ester, the reaction needs to be heated under the reflux for a specific time to produce an equilibrium mixture followed by fractional distillation.
