Introduction
It is a biophysical method widely used for the separation of different components in a mixture of solvents. Components of a mixture are separated through chromatography due to their differential adsorption ability between stationary and mobile phase.
This method is used routinely in chemical laborites, pharmaceuticals and in industry. Though there are different chromatographic techniques, all chromatographic system consists of a stationary phase which in all the cases comprise of a solid surface or a liquid layer adsorbed on a solid surface and a mobile phase which is either liquid or gaseous phase.
So, it is based on the fact that stationary phase which is a stable phase when a mixture is applied to the chromatographic system it binds to the stationary phase and it components get separated by the movement of the mobile phase.
The ability of the chromatography method to separate the components based on their physical properties. It is useful in identification, quantitative and qualitative evaluation and purifications of components of a mixture solution. The physical properties of molecules such as polarity, charge, size and shape play important roles in retention and movement of molecules through stationary phase and mobile phase though affect the separation efficiency.
Brief History
Chromatography method is first used by the Mikhail Tsvet in 1901, he was a botanist and used this method for the separation of the plant pigments (carotenes, chlorophyll and xanthophylls), as these pigments are colourful, so he had given this method the name chromatography.
After that many scientists worked contributed in the development of the chromatography and Archer John Porter Martin and Richard Laurence Millington Synge won the noble prize in 1952. They found that the basic principle of chromatography can be used in different ways to develop more related methods with various applications. Their work led to the development of paper chromatography, partition chromatography and gas chromatography.
Since the time of Tvest, chromatography field advanced greatly leading to the development of efficient methods (HPLC, FPLC) and sophisticated machines which can separate molecules with high efficiency and with minimum human involvement.
Types of Chromatographic methods
There are different chromatographic methods available which can be classified based on (1) stationary phase interaction with solutes, (2) stationary phase bed shape and (3) mobile phase physical states.
Brief description of different chromatography methods based on their classifications.
Chromatography class based on stationary phase interaction with solutes
This type of chromatography consists of different techniques by using the physical properties of the solute for the efficient separation of solution components.
- Adsorption chromatography: The stationary phase is adsorbent which adsorbs the liquid or gaseous mobile phase. The solutes in solution when applied, adsorbed on the stationary phase surface (adsorbent) depend on their interaction with the adsorbent. Liquid or gaseous mobile phase movement helps in the separation of different solutes at different time intervals. So, the mixture components which have greater interaction retained on the surface for greater time and loosely bind components move faster so come out of the column first.
- Partition chromatography: in this type of chromatography components of a mixture are separated by partitioning them between two liquid phases. This technique was first published in the 1940s by Richard Laurence Millington Synge and Archer Martin. Partition chromatography is also called Liquid-Liquid Chromatography and in the case of the gaseous mobile phase, it is called Gas-Liquid Chromatography. The stationary phase consists of a thin liquid layer coated on a solid support, whereas the mobile phase is liquid. The components of the mixture get separated as the mobile phase moves through the stationary phase.
- Ion Exchange chromatography: this method was first used in 1962 for the analysis of work of arts in order to separate the modern art samples from antique art samples. This separation method based on the interaction between differently charged particles in two phases. The stationary phase consists of solid charge particles (resin) which have the ability to interact with solute particles present in the liquid mobile phase. This method is widely used for the purification of proteins.
Chromatography class based on stationary phase shape
- Column chromatography: It is a widely used method as all the chromatography methods used consist of columns except thin layer chromatography. The stationary phase which may be solid particles or liquid layer coated on a solid support is packed in a tube while the liquid mobile phase moves through the stationary phase.
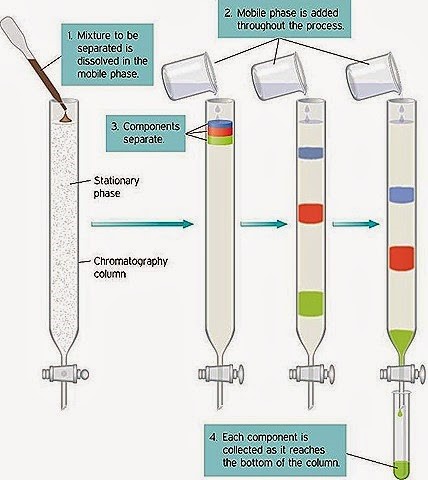
Figure 1: Column chromatography
Mobile phase movements are due to gravity which takes too much time and makes it a lengthy process whereas in many cases pressure develops through pressure pumps so that the mobile phase moves fast. These flash chromatography systems such as HPLC and FPLC make the separation more efficient and faster.
- Paper chromatography: In this technique, a paper that is made up of a polar substance like cellulose, is used as the stationary phase. The sample is placed on the paper as spot at the bottom and then this paper placed in tank which contained the solvent enough that it must touch the base of the paper. As the solvent moves upward the components of the samples separated based on the fact of their different polarity. Nonpolar components move faster and further as compared to polar components that bind with the cellulose.
- Thin-layer chromatography: This technique is very similar to paper chromatography, but the main difference is that instead of a paper, the stationary phase consists of a thin layer of alumina, silica gel or cellulose which is coated on a solid flat support. The mobile phase is liquid and contains a mixture of components that need to be separated. The solvent moved up the plate due to the capillary action. Different components of the mixture move up the stationary phase at a different rate, they get separated. It is more efficient and faster than paper chromatography. It also gives the choice to use different materials as the stationary phase depends on the properties of the mixture to be separated.
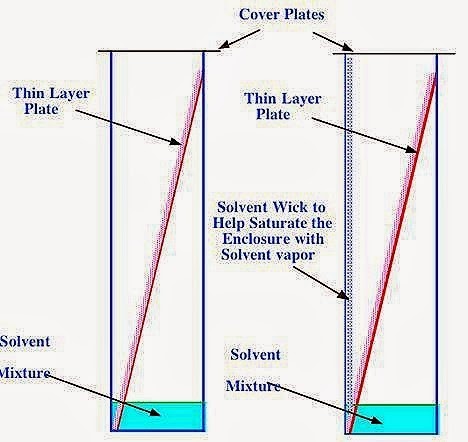
Figure 2: Thin layer chromatography
- Displacement chromatography: This technique was first developed by Arne Tiselius in 1943 and Csaba Horvath advanced the technique by introducing high-pressure pump and equipment. This technique based on the principle that certain solutes interact more strongly with the resin than other solutes present in the solution though displacing other solutes. This type of chromatography results in separation of a particular solute in highly purified form so this method is widely used in the purification of proteins and transuranic elements.
Chromatography class based on the type of mobile phase physical state
- Gas Chromatography: In this method, the mobile phase is a gas which in most cases is helium gas and it is carried out in a column. The stationary phase is tightly packed in the narrow diameter glass tube or in a metal tube. The applied sample passed through the column under controlled temperature gradient and the passing of the gaseous mobile results in separation of the mixture components depend on various factors including the boiling point, polarity and molecular weight of the components.
- Liquid Chromatography: In this technique, the mobile phase is liquid while the stationary phase consists of solid particle packed in a column. The separation of the solutes depends on their interaction with the two phases. In new advance technology very, a small particle used in packing and high pressure is implied to separate the components of the mixture, this new technology is called high-pressure liquid chromatography (HPLC).
- Affinity Chromatography: This method is widely used to purify or separate a specific group of molecules from the solution mixture. In this technique, selective interaction between a solute and a specific molecule is used for separation. These interactions can be between enzyme and its substrate, antibody and antigen, receptor and ligand. The stationary phase contains the interacting molecule, whereas the component which needs to be separated is present in the mobile phase. This type of chromatography is mostly used for the purification of the proteins in which a His-tag is fused with protein. This His-tag interacts with Ni so the stationary phase contains Ni-ions. This method is getting the popularity it is fast, high yield of purified protein and specificity.
Applications
As mentioned above chromatography is done using different methods and it also has many applications in pharmaceuticals, food industry, chemical and life sciences.
In pharmaceuticals chromatography can be used to identify different chemicals based on their sizes and it can also use to observe any impurity in medicines. In chemical industry chromatography techniques used to identify the pollutants in the air as well as contaminations in oils and pesticides.
In food industry it can be used to identify nutritional components of the food and to detect and quantify any additives which used to enhance the taste of food. The detection of spoilage (organic acids produced by any bacteria such pyruvic acid produced by psychotropic bacteria) in food helps in determining the food quality and it is a rapid detection method as compared to bacterial plating which can take several days. It can also use to quantify nutritional components in a specific food.
Chromatography methods are widely used in life sciences.
Gas chromatography is used in analyzing crime scenes by testing the blood and hair samples. Gas chromatography also used to detect the chemical and fluids present in the human body after death such as the presence of alcohols and poisons. Chromatography is used routinely in molecular biology laboratories for the purification of the recombinant proteins and it is also used widely in proteomics and metabolomics research.
Chromatography techniques like EC-LC-MS are used to study oxidation reaction of drug metabolism and used to study pharmaceutically important compounds like lidocaine alprenolol, diclofenac, albendazole and chlorpromazine. Chromatography methods used to study the oxidation of protein and peptide in proteomic studies. In nucleic acid research gas chromatography, MS and LC together with electrophoresis used to study the oxidation of nucleotides, nucleosides and nucleobases.
References
- Chavan, M., Sutar, M., & Deshmukh, S. (2013). Significance of various chromatographic techniques in drug discovery and development. Int J Res Pharmacy Chem, 3, 282-289.
- Snyder, L. R., Kirkland, J. J., & Dolan, J. W. (2011). Introduction to modern liquid chromatography. John Wiley & Sons.
- Roge, A. B., Firke, S. N., Kawade, R. M., Sarje, S. K., & Vadvalkar, S. M. (2011). Brief Review On: Flash Chromatography. International Journal of Pharmaceutical Sciences and Research, 2(8), 1930.
- Coskun, O. (2016). Separation techniques: chromatography. Northern clinics of Istanbul, 3(2), 156.
- Ismail, B. P. (2017). Basic principles of chromatography. In Food Analysis (pp. 185-211). Springer, Cham.
- Mimansha Patel. (2018). Review Article: Chromatography Principle and Applications. International Journal of Pharmaceutical Sciences and Research, 13 (4), 288-293.
- Jandera, P. (2011). Stationary and mobile phases in hydrophilic interaction chromatography: a review. Analytica chimica acta, 692(1-2), 1-25.
