Definition of nitrous acid
Nitrous acid (HNO2) is an unstable weak acid available in cold acidic solution or in the form of its salts or esters. Nitrous acid decomposes to form nitric oxide (NO) and nitric acid (HNO3).
Structure
Nitrous acid is a planar molecule where two oxygen atoms attached with one nitrogen atom by one with single bond and one with double bond. The p orbitals of two oxygens and nitrogen in O-N-O bond are in a same plane and the π bonded electrons are delocalized between these orbitals. As a result, both of these bonds have double bond characteristic and the rotation is restricted around the O-N-O bond. Thus geometrical isomers are possible. The single bonded oxygen bonds with hydrogen in such a way that can make cis or trans isomer in gas phase. Trans form predominates in room temperature. IR spectroscopy shows that trans isomer is more stable than cis by 2.3 kj mol-1.
![Nitrous acid 12 By Ben Mills (Own work) [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/0/02/Trans-nitrous-acid-2D-dimensions.png)
![Nitrous acid 13 By Ben Mills (Own work) [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/f/f9/Cis-nitrous-acid-3D-balls.png)
![Nitrous acid 14 By Ben Mills (Own work) [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/c/cf/Trans-nitrous-acid-3D-balls.png)
Occurrence in atmosphere
Nitrous acid is produced naturally in atmosphere by a heterogeneous reaction of nitric oxide (NO) and water, that controls ozone in the lower atmosphere .
Preparation
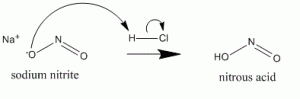
Nitrous acid is unstable and always prepared in situ. It can be made by reacting with sodium or potassium nitrite with hydrochloric acid.
Uses of nitrous acid
Identification of amines
Previously nitrous acid was widely used for the identification of different types of amines. Firstly amines are acidified with hydrochloric acid (HCl), then sodium or potassium nitrite is added to the solution. The nitrous acid is formed in situ to react with amine for the identification.
With primary amines
The observation of colourless and odourless nitrogen gas proves the presence of primary amine. Here lots of different organic compounds are produced with the formation of equimolar nitrogen gas. Measuring the moles of nitrogen gas formed we can get the number of primary amines present in the solution. The product includes an alcohol by replacing the functional group amine (-NH2) with alcohol (-OH).
CH3CH2 NH2 + HNO2 → CH3CH2 OH + N2 ↑ + H2O
With secondary amines
No gas is observed, instead yellow oil of nitrosamine proves the presence of secondary amines. This compound is carcinogens, thus this type of reaction is avoided now a days.
(CH3)2 NH + HNO2 → (CH3)2 N-N=O + H2O
With tertiary amines
Colourless solution proves the presence of tertiary amines. Here an ion is formed by the reaction between tertiary amines and nitrous acid.
Decomposition of nitrous acid
In warm or concentrated solutions, the nitrous acid produces nitric acid, water, and nitric oxide.
3 HNO2 → HNO3 + 2 NO + H2O
Nitrous acid decomposes into nitrogen dioxide, nitric oxide, and water.
2 HNO2 → NO2 + NO + H2O
Nitrogen dioxide reacts further with water to form nitric acid and nitrous acid in aqueous solution.
2 NO2 + H2O → HNO3 + HNO2
The oxidation of nitric oxide by air, gives nitric acid.
2 HNO2 + O2 → 2 HNO3
Reactions
Nitrous acid acts both as oxidizing and reducing agent by losing or gaining electrons.
Nitrous acid as an oxidizing agent
The oxidation number of nitrogen in nitrous acid is +3. When it reacts with H2S it produces nitric oxide (NO) where the oxidation number of nitrogen reduces to +2. Thus here nitrous acid acts as an oxidizing agent.
2HNO2 + H2S → 2NO + 2H2O + S
Nitrous acid as a reducing agent
The oxidation number of nitrous acid increases from +3 to +5 when it reacts with I2 or Br2 in water to produce nitric acid.
HNO2 + Br2 + H2O → HNO3 + 2HBr
HNO2 + I2 + H2O → HNO3 + 2HI
Summary
- Nitrous acid (HNO2) is an unstable weak acid available in cold acidic solution or in the form of its salts or esters.
- Nitrous acid decomposes to form nitric oxide (NO) and nitric acid (HNO3).
- Nitrous acid is a planar molecule where two oxygen atoms attached with one nitrogen atom by one with single bond and one with double bond.
- Nitrous acid can be made by reacting with sodium or potassium nitrite with hydrochloric acid.
- Previously nitrous acid was widely used for the identification of different types of amines.
- Nitrous acid decomposes into nitrogen dioxide, nitric oxide, and water.
- Nitrous acid acts both as oxidizing and reducing agent by losing or gaining electrons.
