Mass Spectrometry Definition
Mass spectrometry is a powerful analytical technique used to know the molecular mass of unknown compound, quantify the known compound and elucidate the structure of a compound. Mass spectrometry convert the sample into gaseous ions with or without fragmentation and then characterize the sample with mass and charge ratios (m/z) and their relative abundance. Mass spectrometry is thus used to know the mass of pure samples as well as complex mixtures.
Basic Priciple
The technique basically used is the effect of ionization energy in the molecule. At first the spectroscopy produces molecular ions (M+) of gas phase sample by electron ionization. The molecular ion (M+) then breaks to form more ions. These ions would like to break in such a way that is comparatively stable or favorable. These ions will break further to form smaller ions. These ions are then separated in mass spectrometry according to their mass and charge ratio (m/z) and are detected in proportion to their abundance.
A mass spectrum is then plotted with the ion abundance versus mass to charge ratio (m/z). The spectrum provide the information about the molecular mass of the sample (M+) and the others peaks gives information about the ions produce from the molecular ion. In spectrum the highest value of m/z (followed by ions containing heavier isotopes) represents the molecular ion of the sample. By analyzing the other peaks of the spectrum (which represents the fragmentation produced from the molecular ion), the structure of the molecule can also me elucidated.

Here a molecule ABC is placed in mass spectroscopy for electronic ionization and firstly molecular ion ABC+ is produced which is then breaks in different ways to form more ions such as AB+, C+, A+, and BC+.
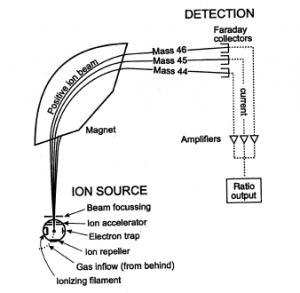
How it Works
In spectrometry, atomic and molecular ions are deflected by magnetic fields. Electrically charged particles are affected by a magnetic field while neutral ones aren’t.
The following steps are involved in spectrometer to display a mass spectrum:
Step 1: Ionisation
The atom or molecule is ionised by electron beam, to remove one or more electrons off to give a positive ion. This is also true even for things like most common negative ions (chlorine, for example) or neutral atoms like argon, for example. Most mass spectrometers work with positive ions.
Step 2: Acceleration
The ions are then accelerated so that they all have the same kinetic energy.
Step 3: Deflection
The ions are then deflected by a magnetic field according to their molecular masses. The lighter they are, the more they are deflected.
The amount of deflection also depends on the number of positive charges on the ions. The more the ion is charged, the more it gets deflected.
Step 4: Detection
The beam of ions passing through the machine is then detected electrically.
Molecular ion
The ion formed by removing one electron from the parent molecule is called molecular ion or parent ion. The molecular ion are usually represented as M+. The molecular ion is the most important peak as it is the molecular weight of the parent molecule.
The other ions are the fragmentation of organic molecules follow certain patterns which are related to the bond strengths and the stability of species formed. The identification of these fragments and their relative intensities in a mass spectrum are used to determine the structure of organic molecules.
Example
- Let us consider the following isomers:
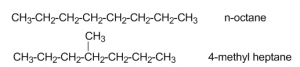
These two isomers which can be distinguished by mass spectroscopy. The methyl group branched at C-4 in the latter compound, is easily broken off to give CH3+. Mass spectrum will show a large peak at m/e = 15.
2. The mass spectrum of toluene or methyl benzene is shown below. The spectrum displays a strong molecular ion at m/e = 92, small m+1 and m+2 peaks, a base peak at m/e = 91 and some minor peaks at m/e = 65 and below.
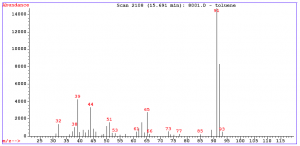
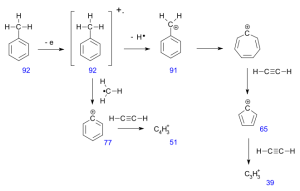
The molecular ion represents loss of an electron and the peaks above the molecular ion are due to isotopic abundance. The base peak in toluene is due to loss of a hydrogen atom to form the relatively stable benzyl cation. This ion rearrange to form the very stable tropylium cation, and gives a strong peak at m/e = 91. The minor peak at m/e = 65 represents a neutral acetylene removed from the tropylium ion and the minor peaks below this arise from more complex fragmentations.
Summary
- Mass spectroscopy involves organic molecules being bombarded by a very high-energy electron beam.
- The peak of highest intensity in a mass spectrum is referred to as the base peak.
- The m/e value of the molecular ion peak showed in mass spectrum is the molecular weight of the compound.
- By analyzing the peaks for other fragments, the structures of organic molecules can be deduced.