Lattice Energy Definition
Ionic compounds are more stable because of their elctrostatic force between the two opposite ions. After the formation of ions, they combine together to form ionic compound. The energy released in this process is known as lattice energy or lattice enthalpy. That means, energy released when a cation and a anion combine together to form one mole of an ionic compound is know as lattice energy or lattice enthalpy. Thus, we can write
A+ + B– → A+B– + Lattice energy
![Lattice Energy 9 By Benjah-bmm27 (Own work) [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/7/72/Lattice-enthalpy-NaCl-3D-ionic.png)
Factors that affect lattice energy
The strength of ionic bond increases with the increase of lattice energy. And lattice energy depends on two factors: size or radius of ions and charge of ions.
a) Radius of ions
As the radius of ions increases, the lattice energy decrease. Below is a graph of the lattice energy of lithium halide. As the size of halide increases down the group, the lattice energy decreases.
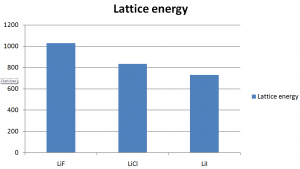
This is because with the increase of size of ions, the distance between their nuclei increases. Thus the attraction between them decreases and finally the less lattice energy released during the process.
b) Charge of ions
Lattice energy increases with increase of charge on the ions because of their more attractive force between them. Thus +2 or -2 ions will release more lattice energy than the +1 or -1 ions.
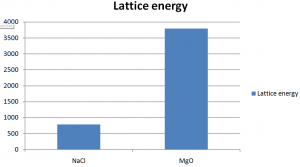
Here we can see that the lattice energy of MgO is much greater than the lattice energy of NaCl.
Calculation of lattice energy
The calculation of lattice energy can be done by using Hess’s law (for this case it is called Born Haber cycle). According to this law the energy change is same for a particular reaction regardless whether the reaction undergoes in one or several steps. So the total energy involved to form a ionic compound from its elements will be same whether it happened in few steps or in one step. For an example, the solid sodium and chlorine gas reacts to form sodium chloride crysltal. The energy change will be same whether it reacts in few steps or in one step. Below is the Born haber cycle for the formation of sodium chloride crystal.
Here the formation of sodium chloride is happening is few steps:
Step 1:
First step involved the formation of sodium atom in gaseous state from its standard solid sodium.
Na(s) → Na(g) ΔHatom = +107 kJ/mol
This is known as atomisation and the energy change during this process is knows as atomisation energy. The energy change involved to get 1 mole of gaseous atom from its standard state is known as atomisation energy. The atomisation energy for this step is +107 kJ/mol.
Step 2:
Second step involved the dissociation of chlorine molecule to form chlorine atoms in gaseous state. The energy of dissociation of bond energy is +122 kJ/mol.
1/2Cl2(g) → Cl(g) ΔHBE = +122 kJ/mol
Step 3:
Third step involved ionisation of sodium in gaseous state to get positively charged sodium ion by losing an electron. The ionisation energy for this step is +494 kJ/mol.
Na(g) → Na+(g) + e– ΔHIE1 = +494 kJ/mol
Step 4:
The fourth step is the addition of electron to the chlorine atom to get negatively charged chloride ion in gaseous state. The electron released for this process is the electron affinity and for this it is -349 kJ/mol.
Cl(g) + e– → Cl–(g) ΔHEA1 = -349 kJ/mol
Step 5:
Fifth and the last step is the formation of sodium chloride from it gaseous ions. The lattice energy released in this process should be negative and need to be calculated.
Na+(g) + Cl–(g) → NaCl(s) ΔHLE = -? kJ/mol
The heat of formation for the whole reaction if it occurs in one step is -411 kJ/mol.
Na(s) + 1/2Cl2(g) → NaCl(s) ΔHf = -411 kJ/mol
According to Hess’s law
ΔHf = ΔHatom + ΔHBE + ΔHIE1 + ΔHEA1 + ΔHLE
⇒ -411 = 107 + 122 + 494 -349 + ΔHLE
⇒ ΔHLE = -785 kJ/mol
So, the lattice energy of sodium chloride is -785 kJ/mol. Similarly lattice energy of any ionic compound can be calculated.
