Ester Definition
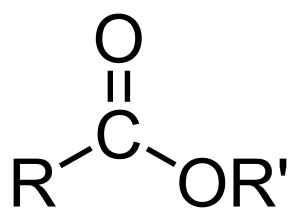
Esters are the derivatives of carboxylic acids in which the hydrogen of carboxylic acid (-COOH) has been replaced by an alkyl group (-R) like methyl, ethyl or a benzene ring like phenyl. The ester functional group may be represented as -COOR.
Esters are found in flowers and fruits which owe their fragrance for these compounds. Esters are very important class of acid derivatives because of their uses in many synthetic products such as perfumes, pesticides, solvents etc.
Nomenclature
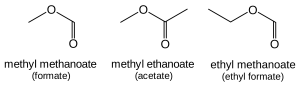
The name of an ester has two part. First the name of the alkyl group R’ which is directly attached with one of the oxygen in RCOOR’. Second part is the name of parent acid with the ending ‘-ic acid’ changed to ‘-ate’ for ester. For example methyl methanoate, methyl ethanoate and ethyl methanoate are the names of following three esters.
Preparation
The preparation of ester is known as esterification. Esterification is an equilibrium reaction to form ester mainly from alcohols and carboxylic acids. Esters can also be made from the reactions between acyl chlorides (acid chlorides) and alcohols, and from acid anhydrides and alcohols.
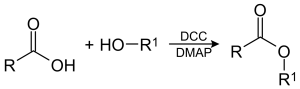
Here carboxylic acid and alcohol reacts to form an ester. Thus this is an esterification reaction. Esterification can occur in three different ways
- From carboxylic acid and alcohol
- From acid chloride and alcohol
- From acid anhydride and alcohol
1. From carboxylic acid and alcohol
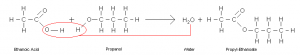
Esters are mainly produced from carboxylic acid and alcohols by heating in presence of acid catalyst. Usually concentrated sulfuric acid is used as a catalyst. This is also know as Fischer esterification reaction. For example ethanoic acid and propanol reacts to form propyl-ethanoate and water.
2. From acid chloride and alcohol
Esters can also be made from acid chloride and alcohol at room temperature. After a vigorous (even violent) reation ester is formed with steamy acidic fume of hydrogen chloride. For example, benzoyl chloride and alcohol reacts to form ester.
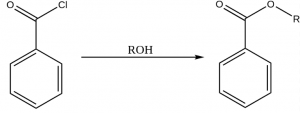
3. From acid anhydride and alcohol
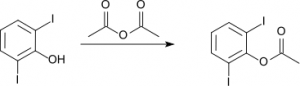
The reaction between acid anhydride and alcohol is comparatively slower than with acid chloride and usually need to warm the mixture to get more esters. For example, 2,6-diiodophenol reacts with acid anhydride to form ester.
Physical properties
Boiling point
Small esters have boiling points, similar to those of aldehydes and ketones with the same number of carbon atoms. Esters are polar molecule like aldehydes and ketones. But they don’t form hydrogen bonds. Thus the boiling point of esters are lower than the acid with same number of carbon atoms.
| Molecule | Type | Boiling point (oC) |
| CH3COOCH2CH3 | Ester | 77.1 |
| CH3CH2CH2COOH | Carboxylic acid | 164 |
Solubility
The small esters are fairly soluble in water but the solubility falls with increasing carbon chain length. Although esters can not attach themselves with intermolecular hydrogen bond, but they can make hydrogen bond with water molecules.The lone pair of oxygen in ester molecule can make hydrogen bond with the partially positive hydrogen atom in water molecule.
As increasing the carbon chain length, the hydrocarbon part of the ester molecule get in the way to form hydrogen bond between ester and water. Thus the solubility decreases.
| Name of the ester | Formula | Solubility (g per 100 g of water) |
| Ethyl methanoate | HCOOCH2CH3 | 10.5 |
| Ethyl ethanoate | CH3COOCH2CH3 | 8.7 |
| Ethyl propanoate | CH3CH2COOCH2CH3 | 1.7 |
Characterization
Esters are generally characterized by gas chromatography, because of their volatility. In the IR spectrum, esters show a characteristic intense sharp band in the range between 1730–1750 cm−1 for νC=O.
Chemical properties
The carbonyl carbon in ester acts as a weak electrophile and is attacked by strong nucleophile like amines, alkoxides, hydride sources etc. Thus ester gives nucleophilic substitution reaction like acid halides and anhydrides.
Hydrolysis of ester
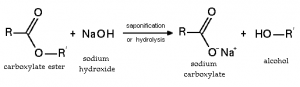
Upon hydrolysis by heating with water in presence of acid (H2SO4 or HCl) as a catalyst ester gives parent carboxylic acid and alcohol. The reaction is reversible.

The hydrolysis of ester can also be achieved by heating with reflux in presence of aqueous solution of strong base like sodium hydroxide.
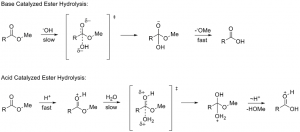
The mechanism of acid and base catalyzed hydrolysis are given below:
Summary
- The ester is a functional group, which may be represented as -COOR.
- Esters can be prepared from alcohols and carboxylic acids, acyl chlorides (acid chlorides) and alcohols, or from acid anhydrides and alcohols.
- The boiling point of esters are lower than the acid with same number of carbon atoms.
- The small esters are fairly soluble in water but the solubility falls with increasing carbon chain length.
- Esters are generally characterized by gas chromatography and the IR spectrum shows a characteristic peak between 1730–1750 cm−1 for νC=O.
- Upon hydrolysis by heating with water in presence of acid (H2SO4 or HCl) or base (NaOH) as a catalyst ester gives parent carboxylic acid and alcohol.
