In chemistry, an equivalence point is a term that is used while performing titration. It applies to any acid-base or neutralization reaction technically.
Definition:
The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically.
In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. The number of moles of titrant i.e. standard solution is equal to the moles of a solution having an unknown concentration. It is also known as the stoichiometric point because it is a point where the moles of acid is equal to the moles of the base that are needed to neutralize the solution. Note that acid to base ratio doesn’t need to be 1:1. This acid-base ratio is explained by the balanced acid-base chemical equation. Indicators can be used for this purpose, for example, methyl orange or phenolphthalein.

The equivalence point cannot be taken the same as the endpoint of a titration. The endpoint is referred to as the point at which the used indicator changes its color. These color changes appear after reaching the equivalence point. If we use the endpoint to determine equivalence, it will induce an error.
Methods of Determining the Equivalence Point:
There are different methods for the determination of the equivalence point. They are discussed as follows:
- pH indicator
- Conductance
- Color Change
- Precipitation
- Isothermal calorimetry
- Thermometric titrimetry
- Spectroscopy
- Amperometry
pH indicator:
a pH indicator is a chemical substance that changes its color in reaction to any chemical change. An acid-base indicator, for example, phenolphthalein changes its color which depends on its pH. Redox indicators can also be used for this purpose. Initially, just a single drop of indicator solution is added to the titration. The change in color will show that the endpoint has been attained. This will be an estimation of the equivalence point.
Conductance:
The electrical conductivity of a solution is affected by the ions. Hence the conductivity changes when they react with each other. (for example, while performing an acid-base titration, the H3O+, and OH- ions reacts and form neutral water, H20 solution). Conductance is relatively a difficult method to operate, especially when the ions that are present in the solution can participate in conductivity. Conductance can be used for a few acid-base reactions.
Color change:
At the equivalence point, the solution will change its color naturally without any addition of an indicator in some reactions. This may be observed in transition metals where the oxidation state consists of different colors.
Precipitation:
During titration, the precipitate will form if the reaction forms a solid. A good example of precipitation is the reaction between silver, Ag+, and Chlorine, Cl- that results in the formation of an insoluble salt, Silver Chloride, AgCl. Unexpectedly, this makes it very hard to determine the endpoints accurately because of particle size, the rate of sedimentation and color makes it very difficult to see. This is the reason why precipitation titration is done as back titrations.
Isothermal Calorimetry:
The determination of the equivalence point is done by calculating the amount of heat that is produced or absorbed by using a device known as an isothermal titration calorimeter. This type is usually used in titrations that involve biochemical reactions i.e., as enzyme binding.
Thermometric Titrimetry:
Thermometric titrimetry is an exceptionally multifaceted technique. Here the equivalence point is determined by measuring the rate of temperature change produced by a chemical reaction. This property differentiates it from calorimetric titrimetry. Because thermometric titrimetry is a relative technique, it is not necessary to perform the titration under isothermal conditions. This type of titration can be conducted in plastics or even in glass vessels. To prevent stray draughts, these vessels are usually enclosed by causing any noise that disturbs the endpoint. Because of the ability of this type of titration to be conducted under ambient conditions, they are appropriate for routine process and quality control in the industry. The temperature will either increase or decrease during the titration process, depending on whether the reaction taking place between the titrant and analyte is exothermic or endothermic. It titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. an increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. The equivalence point can be observed precisely by employing the second derivative of the temperature curve. The software which is used in a modern automated thermometric titration system consists of regular advanced digital algorithms so that the noise generating from highly sensitive temperature probes does not cause any interference with the appearance of a regular, uniform second derivative peak that describes the endpoint. This technique has the ability of very high precision and the coefficient of variance (CVs) of less than 0.1 are usual. The latest thermometric titration temperature probes have a thermistor that forms one arm of a Wheatstone bridge. the best thermometric titration system can resolve temperature to 10-5 K if coupled to high-resolution electronics. If the temperature changes while performing titration become as little as 0.001K a sharp equivalence point will be obtained. Where there is an enthalpy change, this technique can be applied necessarily to any chemical reaction in a fluid, though the reaction kinetics plays an important role in calculating the sharpness of the endpoint. This point of titrimetry has been substantially applied to acid-base, EDTA, REDOX and precipitation titration. Important examples of precipitation titration include:
- Sulfate titration with Barium ions
- Phosphate titration with Magnesium in ammonium solution
- Chloride titration with silver Nitrate
- Nickel titration with Dimethylglyoxime
- Fluoride titration with Aluminum (as K2NaAlF6)
As non-aqueous titrations can easily be carried out as aqueous titration because the temperature probe does not require to be electrically connected to the solution as it is required in potentiometric titrations. Solutions that are highly turbid or colored can be easily analyzed by thermometric without any further treatment of a sample. The probe is maintenance-free. Now a day by using the latest and highly précised stepper motor driven burettes, thermometric titrations are completed within a few minutes, making this technique a perfect choice where high production in a laboratory is needed.
Spectroscopy:
in this type, spectroscopy is used to determine the equivalence point if the spectrum of the reactant, product or titrant is known. A specific amount of the product and reactant is used to find the equivalence point. A very low level of the free titrant’s presence can also be determined. In short, this method is used to determine the existence of semiconductors.
Amperometry:
Amperometry is a detection technique that is used to measure the change in the current. Amperometry is mostly used in those titrations where the excess titrant can be reduced. This method is helpful while titrating a halide with Ag+ because the formation of precipitates will not be affected.
Examples of equivalence point:
- The reaction of a strong acid with a strong base:
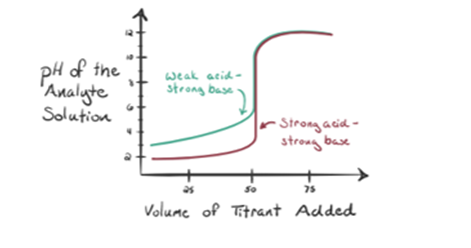
Let suppose hydrochloric acid HCl (a strong acid) is taken as an analyte and sodium hydroxide NaOH (strong base) is taken as a titrant. If we plot a graph between analyte pH and a titrant NaOH which can be added from the burette, a titration graph will be formed like is given below:
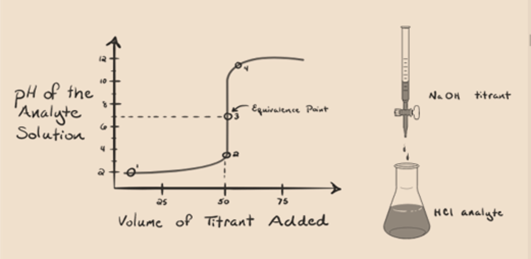
Figure 1.1
The above figure 1.1 shows at point 1 that when no base like NaOH is added. The pH of the analyte is low as it mostly contains H3O+ from the separation of HCl.

As sodium hydroxide NaOH is added drop by drop, it starts getting absorbed by OH– slowly that is produced by dissociation of NaOH. The produced analyte will be acidic due to the dominant presence of hydronium ions, H3O+.
In figure 1.1 point 2 indicates the time point at which the pH is recorded just before the neutralization takes place completely. While point 3 shows us the equivalence point. Here the moles of sodium hydroxide added is equal to the moles of the hydroxyl chloride in the analyte. This is the point where hydronium ions, H3O+ are completely neutralized by hydroxyl ion, OH–. The pH of the solution is neutral i.e. pH=7 because it has salt, NaCl and water H2O.
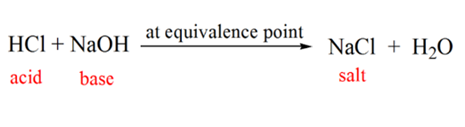
Point 4 of figure 1.1 shows that as we keep on adding NaOH, the pH of the solution starts becoming basic because of the complete neutralization of the HCl. Now there are plenty of OH– ions are present in the solution which dissociates from NaOH.
- The reaction of a weak acid with a strong base:
Let’s consider a weak acid, an acetic acid CH3COOH and a strong base sodium Hydroxide NaOH as a titrant. If we plot a graph between the pH of the analyte and the volume of NaOH, we will get a titration curve as shown below.
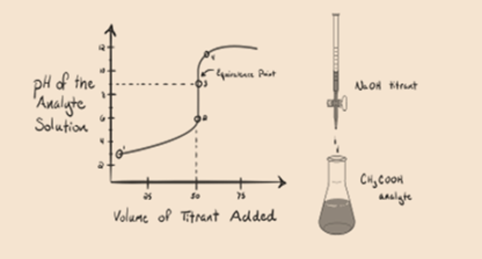
Figure 1.2
Figure 1.2 shows at point 1 that NaOH is not added yet, so right now the pH of the analyte is low as it mostly contains H3O+ when CH3COOH dissociates. But as acetic acid is a weak acid, hence the initially its pH will be higher.

As we keep on adding NaOH dropwise, H3O+ will start getting consumed by OH– slowly. This will be produced by the dissociation of NaOH. But here the analyte is still acidic due to the presence of H3O+ ions mostly.
In figure 1.2 point 2 indicates the pH recorded at a time just before a neutralization takes place completely.
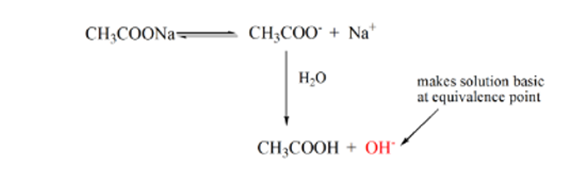
Point 3 represents the equivalence point. This is the point where moles of NaOH is equal to the moles of CH3COOH in an analyte. Hydronium ions are completely neutralized by hydroxyl ions. The solution will then contain CH3COONa salt and H2O.

Note:
You can notice a difference here as compared to a case 1 with a having a strong acid titrated against a strong base. In this case (weak acid and a strong base), the pH is not neutral at a point of equivalence. The solution is having a pH~9 at the equivalence point. Let’s figure out the reason below.
From the above equation, it is shown that the solution contains CH3COONa at the equivalence point. This dissociates into a sodium ion and acetate ions. Acetate ion is the conjugate base of the weak acid CH3COOH. Hence, CH3COO– is relatively a strong base (i.e. weak acid has a strong conjugate base) therefore they react with water to produce hydroxide ions that increase the pH to near to 9 at the point of equivalence.
Point 4 in figure 1.2 shows that when sodium hydroxide is in greater amount, the gained titration curve will be identical to HCl-NaOH.
- Strong acid with a weak base:
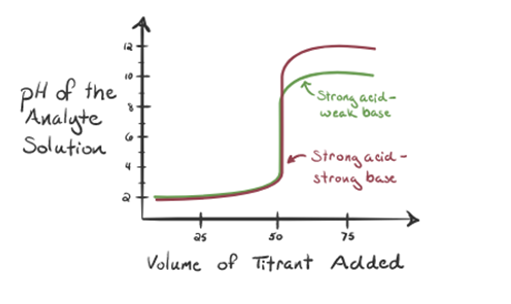
Here, let’s consider hydrochloric acid as a strong acid as an analyte and ammonia as a weak base as a titrant. If we plot a graph between the pH of the analyte solution and volume of the titrant NH3, we will get a titration curve as given below:
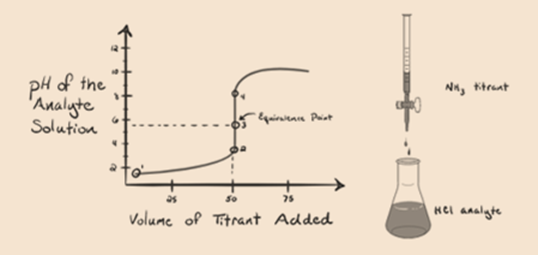
Figure 1.3
In figure 1.3 point 1 shows that when no ammonia is added, the pH of the analyte will be low as it mostly contains H3O+ from the dissociation of Hydrochloric acid.
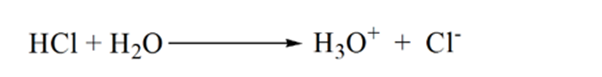
As ammonia is added drop by drop, H3O+ starts to get consumed by ammonia slowly. The analyte will be still acidic due to the majority of H3O+ ions.
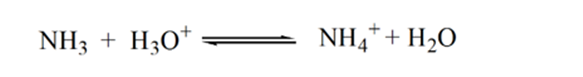
Point 2 in figure 1.3 indicates that the pH is recorded at a point just before a neutralization takes place completely.
Point 3 shows the equivalence point. Here the number of moles of added NH3 is equal to the moles of HCl in the analyte. The hydronium ions are fully neutralized by NH3.
Note:
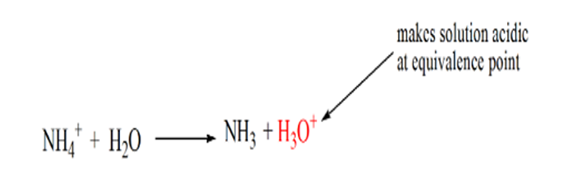
It is noticed that in the case of the weak base against a strong acid, the pH is not neutral at the point of equivalence. Hence the solution that is achieved will be acidic having a pH around 5.5 at the point of equivalence.
The reason for this is that at a point of equivalence the solution has only ammonium ions NH4+ and Chloride ions, CL-. As the ammonium ions are the conjugate acid of a weak base i.e. NH3. Therefore, NH4+ is a strong acid and thus NH4+ will react with water to produce hydronium ions which makes the solution acidic.
Point 4 shows that after achieving an equivalence point, we will keep on adding ammonia and when in excess, the pH will start increasing. Ammonia is a weak base so its pH is above 7 but it as lower as compared to a strong base NaOH shown in case 1.
- Weak base with a weak acid:
Let’s consider ammonia, a weak base as an analyte and an acetic acid which is a weak acid as a titrant. If we plot a graph between the pH of the analyte solution versus the volume of the acetic acid as a titrant, we will get a titration curve as below:
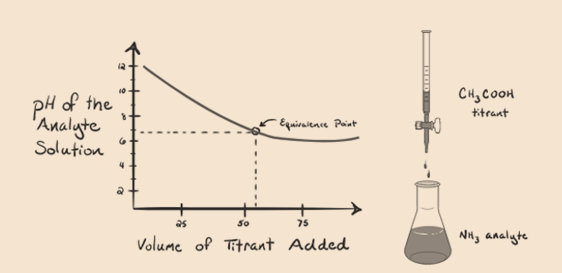
Figure 1.4
We have noticed that there is no steep in this graph plot. This is what we call a “point of inflection” at the point of equivalence. The absence of the required steep change in pH does not give us much information through such a curve.
References:
https://www.thoughtco.com/definition-of-equivalence-point-605101
