Introduction
Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under standard conditions. Electrode potential is also reduction potential and it is calculated for many elements by comparing with standard electrode potential of hydrogen electrode which is also called standard hydrogen electrode (SHE).
In order to understand the electrochemical series and how they arranged, first, we will consider the explanation of some other terminologies.
Oxidation and reduction reactions
Oxidation reactions are those reactions in which an element or ion loses one electron or more than one electrons. In the process of the losing electron that element gets oxidized. Such element is called a reducing agent as to when it loses one or more electron which will be accepted by another element. So in the process of oxidation it reduces the other element so it is called reducing agent.
Reduction reaction are those reaction in which an element or ion gains one electron or more than one electron. The element or ion which receives the electron or electrons get reduced. Such element is called oxidizing agent as in the process of gaining electron or electrons it is oxidizing other element which is losing electron easily. Opposite to a reducing agent, an oxidizing agent itself is reducing while oxidizing the other element.
Electrode potential
Electrode potential is the difference between the potential between the metal electrode and its ionic solution. It depends on the types of metal, ions concentration in the solution and temperature. Metal electrode dipped in its ionic solution either accepting electrons or they are giving electrons in solution mean they are either in oxidizing or reduction condition. One metal electrode dipped in its ionic solution is called half-cell. So it is difficult to calculate the electrode potential of an element from half-cell. This half-cell is completed by adding platinum hydrogen electrode and a salt bridge, while the electrode potential is measured by voltmeter. As mentioned above hydrogen is considered as standard and a system which consisted of two hydrogen electrodes have zero reduction potential (Eo). When replaced the one electrode of hydrogen with any other metal electrode and the change in potential in term of volts can be observed due to different tendency of the metal in accepting or giving electrons as compared to the hydrogen electrode. A cell consists of two hydrogen electrode in equilibrium as potential of gaining and losing the electron is same so reduction potential is zero. But when this equilibrium is shifted to left in a cell, where one hydrogen electrode is replaced with other metal electrode its potential is measured as negative, whereas if metal electrode shifts the equilibrium towards right it is considered as positive reduction potential.
Current flow can be detected by the defection produce in the voltmeter. If the deflection occurs at one side then the flow of current in the opposite direction. So, if the voltmeter deflect towards the metal electrode it means that electron moves towards the hydrogen electrode and the metal acts s anode while hydrogen electrode is cathode. But in a condition, where voltmeter deflect towards hydrogen electrode, it means electrons move towards metal electrode which is in this case act as cathode and hydrogen electrode is anode.
Characteristics of electrochemical series
So as mentioned above electrochemical series is an arrangement of the elements according to their standard electrode potential in specific conditions. Hydrogen is taken as a standard electrode which electrode potential is zero so the elements which are positioned above the hydrogen have negative reduction potential, whereas elements which are arranged below the hydrogen have positive electrode potential. So the elements above the hydrogen are strong reducing agents than hydrogen, whereas those below hydrogen are strong oxidizing agent than hydrogen.
Elements are arranged in an order in which their strength as reducing agent is decreasing from top to bottom, whereas strength of oxidizing agent increasing from bottom to top. The element on the top the series is a strongest reducing agent which means it can lose electron easily than any other element and it is also most active metal. The element at the bottom of the series is a strongest oxidizing agent which means it can gain electron easily than any other element and it is also most non-active metal.
The negative sign of standard electrode potential of a metal showed that when this metal electrode is combined with hydrogen electrode it act as anode and oxidation reaction takes place at this electrode. While the positive sign of standard electrode potential showed that this metal electrode in combination with hydrogen electrode act as cathode and reduction reaction takes place at this electrode.
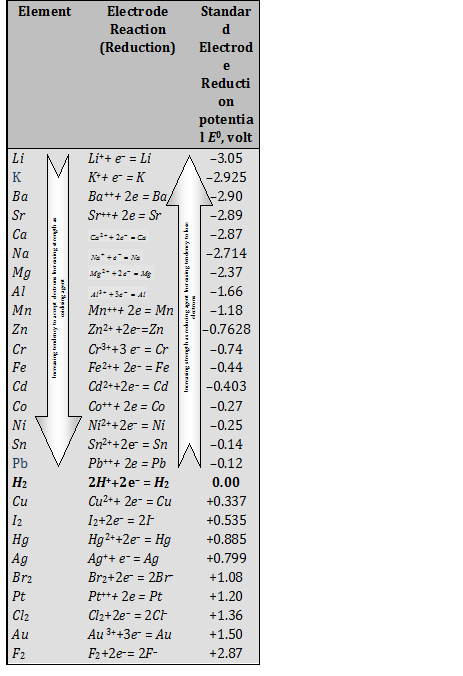
Fig. 1 : Standard electrode potential series.
Applications
Electrochemical series can be used to predict various properties of an element based on their position in the series relative to the hydrogen electrode.
- Electrochemical series can be used to predict which elements is more reactive and which is not. The reactivity of the metals depends on the fact that how easily they lose one electron or more than one electron. Element which is placed on the top of the series have highest negative reduction potential which means it loses lose electron or electrons easily than other elements thus more reactive. The reactivity of the metals is gradually decreasing from top to bottom and the elements placed above the hydrogen standard potential are more reactive as they have negative reduction potential than elements placed below hydrogen which have positive reduction potential. Alkali metals or alkaline earth metals placed above the series as they have highest negative reduction potential thus most reactive and can react with cold water while releasing hydrogen gas. They can also easily combine with acids and easily react with compounds which can accept electrons. Metals (Fe, Pb, Ni, Co etc.) which lie below them but have negative reduction potential are less reactive and they cannot react with cold water but react with water at high temperature. Other metals such as Cu, Au, etc. which have positive reduction potential show very low reactivity and cannot react with water even high temperature.
- Like reactivity of metals, electropositive property of metals is also depends on the tendency of a metal to lose electron or more than one electrons. Metal with highest negative reduction potential consider to have strong electropositive character. Similar to metal reactivity, their electropositive character is also decreasing from top to bottom in the series. Based on the range of stand electrode potential values we can classify metals into three groups. Strong electropositive metals are those which have standard electrode potential from -2.0 volts or above it. So the alkali metals and alkaline earth metals are classified as strong electropositive metals. The second group is named as moderate electropositive metals which have standard electrode potential in range from 0.0 to -2.0. Metals like Al, Zn, Ni, Co and Fe are placed in this group. The third and final class is weak electropositive metals which have positive standard electrode potential and placed below hydrogen. Metals like Cu, Hg and Ag placed in this group.
- Electrochemical series is also useful in determining the ability of metals to displace other metals, the ability of nonmetals to displace others nonmetals, displacement of hydrogen from weak acids and displacement of hydrogen from water. The metal which has low reduction potential displace the metal easily with high reduction potential. It means the metal placed above the electrochemical series can displaced the metal from its solution which is placed below it. In contrast to this, in the case of nonmetals, a nonmetal with higher reduction potential can displace the nonmetal with low reduction potential. So, the nonmetal elements with higher reduction potential place below in series can displace nonmetal placed above it easily. The other property of the metals which can be determined using the electrochemical series is how easily they displace hydrogen from the acid solution. The metals with low reduction potential when mixed with a weak acid solution that contains free H+ ions, release electrons that are readily accepted by free H+ ions to form hydrogen gas which is then liberated from the solution and form the respective salts of metals in the process. So, the elements placed above the hydrogen in electrochemical series have more ability to displace hydrogen from acids than elements placed below it and the tendency of elements in liberating hydrogen from acids is decreasing from top to bottom in the series. Similarly, metals with high negative reduction potential can even liberate hydrogen gas from cold water but metals like Mg, Fe with low negative reduction potential liberate hydrogen from hot water. The ability of the metals in liberating hydrogen from water also decreasing from top to bottom.
- Electrochemical series is also helpful in determining the stability of oxides form by the metals. The metals which are more electropositive in nature form more stable oxides. While the stability of the metal oxides decreases from top to bottom which means that metals with positive reduction potential form less stable oxides than metals which have negative reduction potential. Metals with high positive reduction potential which are placed below Cu form highly unstable oxides as they decompose easily with increasing temperature.
- By determining the oxidizing and reducing ability of the elements which participate in a reaction form electrochemical series, is very helpful in determining the nature and feasibility of a reaction. As mentioned above the oxidizing ability of metals decreasing from top to bottom and reducing the ability of metals increasing from bottom to top. So, a reaction between a metal having negative reduction potential with a metal having positive reduction is more feasible than both metals having either positive or negative reduction potential.
- The ability to displace a metal that is less electropositive in nature by a metal that is more electropositive in nature is used to extract metals like Ag and Au from solution using the cyanide process.
References
- Kosky, P., Balmer, R. T., Keat, W. D., & Wise, G. (2015). Exploring engineering: an introduction to engineering and design. Academic Press.
- Vanysek, P. (2000). Electrochemical series. CRC handbook of chemistry and physics, 8.
- Importance and Applications Of Electrochemical Series (with Examples)
- https://www.curlyarrows.com/importance-electrochemical-series
- What Does Electrochemical Series Mean?
- Veerendra – https://www.aplustopper.com/electrochemical-series-mean/
- Application Of Electrochemical Series
- https://www.ukessays.com/essays/biology/application-of-electrochemical-series-biology-essay.php
- Importance Of Electrochemical Series
- https://www.ukessays.com/essays/biology/understanding-the-importance-and-application-of-electrochemical-series-biology-essay.php
