The word equilibrium is derived from the Latin word equilibrium which means “an equal balance”. Therefore, we can say that it is a state in which the opposing forces are balanced or a system that is not changing with time i.e. no net force is acting.
Equilibrium is divided into two types: static equilibrium and dynamic equilibrium. A static equilibrium is defined as a state of the body in which the resultant force exerted on an object is zero and the object is stationary.
Dynamic equilibrium is an important concept of chemistry.it is important to understand how can something be dynamic and at equilibrium at the same time.
What is a dynamic equilibrium?
Chemical reactions can either go in one direction or both directions i.e. forward and reverse. The forward reaction is those that go in one direction while the ones that go in two directions are called reversible reactions. The direction is identified by the arrows given as follows:
H2O(l) ⇌ H+(aq) + OH–(aq)
Therefore, the dynamic equilibrium can be defined as:
A chemical reaction in which the rate of the reactants is equal to the rate of backward products.
In other words,
A reaction is said to be at dynamic equilibrium when the reactants are converted into products and the products are converted to reactants at an equal and constant rate. So the equilibrium is the state of equal and opposite rate but with an unequal concentration.
Explanation of dynamic equilibrium:
Consider an object is moving at a constant speed that balances all the forces on that object. It will show a dynamic equilibrium when a horizontal force is applied to an object and cause it to move on a horizontal surface with a constant speed. Here the applied force is in the direction of motion while a friction force is produced that is in the opposite direction and balances the applied force-producing no unbalanced force on an object. But as the object is in motion so it continues to move with a constant speed as shown in the figure below:
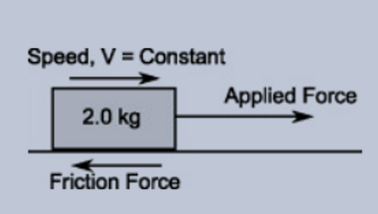
Figure 1.1 showing a wooden piece sliding on a smooth surface
Relationship between Equilibrium and Rate Constants:
Consider the simple reaction as follows:
A ⇌ B
Where
A= reactants
B= products
Therefore, the equilibrium constant Keq can be described as:
Keq= [B] eq/ [A] eq
Where
[A]eq = the reactants at equilibrium conditions
[B]eq = the products at equilibrium conditions
Hence, the rate of reaction is given by:
d[A]/dt = -kf [A] + kb [B]
Where
Kf= the rate constant for the forward reaction
Kb= the rate constant for the backward reaction
The equilibrium constant can also be calculated by dividing the rate constant of the forward reaction by the rate constant of the reverse reaction:
Keq = kf/kb
Where A and B are in equilibrium.
Factors affecting an equilibrium:
Following are the factors that affect the equilibrium:
- By changing the concentration of reactants or products
- By changing the pressure of a gaseous equilibrium
- By changing the temperature
Concentration changes:
If the amount of reactant is increased, the amount of product also increases to maintain an equilibrium. On the other hand, if the amount of reactants decreases, the amount of products also decreases.
Concentration Increases
A + B ⇌ C + D
As the concentration of A increases
the equilibrium moves to the right
Concentration Decreases
A + B ⇌ C + D
As the concentration of A decreases,
the equilibrium moves to the left
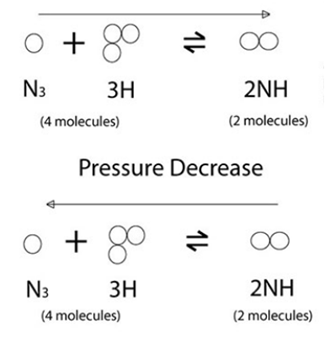
The Pressure Change:
The change in the direction is dependent on the total number of gas molecules present on each side of the equation. If the pressure is increased there will be an increase in the number of molecules created with the least number of gas molecules available. If the pressure is decreased, there will be an increase in the number of molecules created but at the side with the most number of gas molecules available.
Pressure Increase
As the pressure increases, the equilibrium moves to the right.
As the pressure decreases, the equilibrium moves to the left.
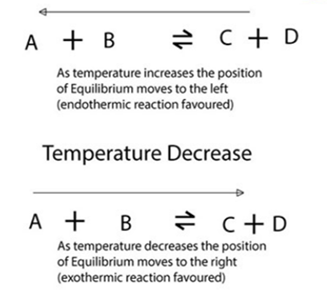
Temperature changes:
If the temperature of the system is increased, the equilibrium will move to bring it down and the reaction will try to absorb the heat. If the temperature is decreased, the system will move to produce the heat.
Temperature Increase
The action of Catalyst:
the catalyst will only help to speed up the reaction to reach equilibrium but will not take part in changing the position of equilibrium.
Equilibrium and Le Chatelier’s Principle:
Equilibrium can be described by Le Chatelier’s Principle. The equilibrium position and the kinetics of a reaction change depend on the properties of reactants and products. The equilibrium transfers towards one side or the other depending on the volume, concentration, pressure, and temperature. Le Chatelier’s principle can’t be confused with dynamic equilibrium as they are similar but have distinct concepts. Le Chatelier’s principle explains how equilibrium can be changed while dynamic equilibrium describes how an equilibrium behaves.
Examples of Dynamic equilibrium:
- Let’s suppose you prepare a saturated solution with sodium chloride, NaCl. Then if you add solid crystals of NaCl, the NaCl will dissolve and recrystallize within the solution at the same time. Therefore, the reaction NaCl(s) ⇌ Na+(aq) + Cl–(aq), will said to be in dynamic equilibrium when the rate of dissolution of NaCl will be equal to the rate of recrystallization.
- If we consider a reaction between nitrogen dioxide with carbon monoxide, by attaining dynamic equilibrium it will give nitrogen oxide, NO and carbon dioxide. As it will be in dynamic equilibrium, so during its reverse reaction, nitrogen oxide and carbon dioxide react to form nitrogen dioxide and carbon monoxide.
NO2(g) + CO(g) ⇌ NO(g) + CO2(g)
- Dynamic equilibrium can also occur in a single-phase system. Let’s consider an example with acid-base equilibrium such that the dissociation of acetic acid takes place in an aqueous solution as shown below:
CH3CO2H ⇌ CH3CO2– + H+
Here, in this case, some protons will be released from acetic acid molecules during a forward reaction while the backward reaction tells us the formation of an acetic acid molecule when a proton is accepted by an acetate ion. Equilibrium is achieved when the sum of reactants on the left side of the equation is equal to the sum of products present on the right-hand side. It means the rate of forwarding and the backward reaction is equal to each other. The formation of the chemical complexes shows a dynamic equilibrium and the concentrations are rules by the stability constants of complexes.
- Civil engineers use dynamic equilibrium during the construction of bridges or buildings. They have to consider all the forces that will be applied to these structures. They maintain the balance of the structure against the exerted forces by calculating the applied forces and this maintain a state of equilibrium.
- Dynamic equilibrium plays an important role in warm-blooded species including ourselves. The homeostasis keeps the body temperature constant. When our body gets too hot then our body helps us to cool us down by a process of sweating. Sweating allows the heat to escape through blood vessels neat to the skin surface to make us feel cool. When our body gets too cold, we start shivering to generate metabolic heat. This results when capillaries become small in size to retain heat and keeps blood away from arms and legs to maintain the core body temperature.
- Consider another example of a bathtub that is filled with water up to a certain level. Take out the plug from the tub and allow the water to keep on adding through a tap. The water that is going out of the tub will be equal to the water that is coming into the tub through a tap. Hence we will observe that the amount of water in the tub will remain at a constant level, while there is a fluent amount of water being added to the tub system. But if we change the system such as the flow from either end of the tub, we will observe that the level of the water will also change depending on how much change will occur in the system.
- In economics the example of the dynamic equilibrium is explained as if you are in a free market, then according to the theory, the price of a product remains the same. This will be possible only when the buyers will buy all the products that they want and the sellers will sell all they want to sell. If the conditions of supply and demand changes, the prices will either decrease or increase.
An Equation that can never be an example of dynamic equilibrium:
4Fe(s) + 6H2O(l) + 3O2(g) changes to 4Fe(OH)3 (s)
This equation tells us about the formation of rust. We will observe that it will never be in dynamic equilibrium because the arrow goes only in the forward reaction that is one way which shows that the rusty object can never come back to its original form.
References:
https://blog.prepscholar.com/what-is-dynamic-equilibrium-definition-example
https://anotherequilibriumsite.weebly.com/everyday-examples.html
https://www.thoughtco.com/definition-of-dynamic-equilibrium-605052
https://blog.prepscholar.com/what-is-dynamic-equilibrium-definition-example
