History
Initially, the term covalence concerning bonding was used in 1919, by Irving Langmuir in an American journal known as the Journal of the American Chemical Society under the title of “The Arrangement of Electrons in Atoms and Molecules.” Langmuir wrote in his journal that “we shall denote by the term covalence the number of pairs of electrons that a given atom shares with its neighbors.”
The idea of covalent bonding is very old. Before 1919, several years back, it was known to Gilbert N. Lewis, who in 1916 explains the sharing of electron pairs between atoms. Lewis introduced the lewis dot structure, or Lewis notation or electron dot notation, in which the valence electrons i.e. those present in an outer shell was represented by dots around the atomic symbol and the pairs of electrons that were located between the atoms were represented by the covalent bonds. The multiple pairs represent multiple bonds for example double bonds and triple bonds.
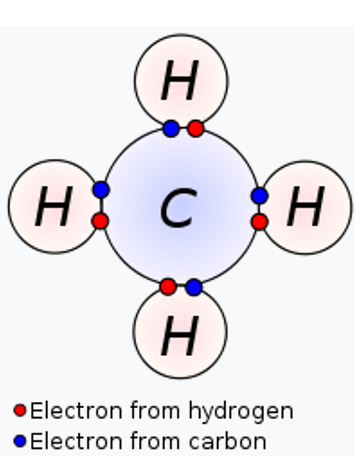
The old concept of a covalent bond has been represented by using Methane molecule. A covalent bond is represented in the Lewis structure that shows electron sharing between atoms.
Covalent bond and its types
A covalent bond which is additionally known as a molecular bond is a chemical bond that involves the sharing of electron pairs between atoms.
Covalent bonds are mostly formed between two non-metals. For example, water, where hydrogen (H) and oxygen (O) forms a bond to make H2O. Here, the oxygen atom shares one electron with each hydrogen atom. It shows that the oxygen has a bit positive charge and that of hydrogen has a small negative charge which as a result are attracted to each other due to the presence of electromagnetic force.
Atomic orbitals have specific directional properties that lead towards different types of a covalent bond. Sigma (σ,) bonds are the strongest type of covalent bonds and this is due to head-on overlapping of orbitals on two different atoms. A single bond is normally called a sigma bond. The pi (π) bonds are relatively weaker and this is due to overlapping between p (or d) orbitals. A double bond between the given two atoms consists of one sigma and one pi bond while a triple bond consists of one sigma and two pi bonds.
Definition of a double covalent bond:
A double covalent bond is the type of chemical bond in which two electron pairs are shared between the two atoms.
This type of covalent bond includes four bonding electrons between atoms rather than the usual two bonding electrons that are involved in a single bond. Double covalent bonds are considered reactive bonds because of the presence of a large number of electrons. The double bonds are shorter and much stronger than single bonds.
Representation:
Double covalent bonds can be represented as two parallel lines. A sign of equal is used to show a double bond in a formula. In the mid-19th century. A Russian chemist Alexander Butlerov tells us the structural formula of a double covalent bond.
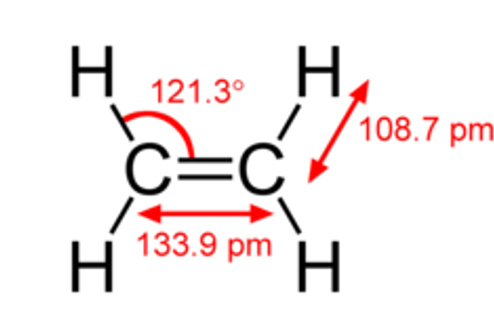
Geometrical representation of ethylene
Formation of a double covalent bond:
Double covalent bonds are created by the sharing four electrons of the two atoms in order to fulfill the octet rule. The octet rule tells that the atoms will share, lose or gain their electrons to achieve eight valence electrons. Double covalent bonds are usually made from nonmetals like carbon, nitrogen, and oxygen.
Electrons move freely and very fast around an atom. Depending on the energy level and closeness to the nucleus, the electrons will stay within a certain shape. The specific shape gained by the electrons is called electron orbitals. There are four shapes of electron orbitals namely s, p, d, and f. in double covalent bonds s and p orbitals are involved.
Some atoms donate more electrons while some donate much less to a double covalent bond. They shuffle themselves around the present electrons to share four electrons.
Examples with simple molecules
Oxygen, O2:
The two atoms of oxygen can gain a stable structure by sharing two pairs of electrons as shown in a figure below.
Carbon dioxide, CO2:
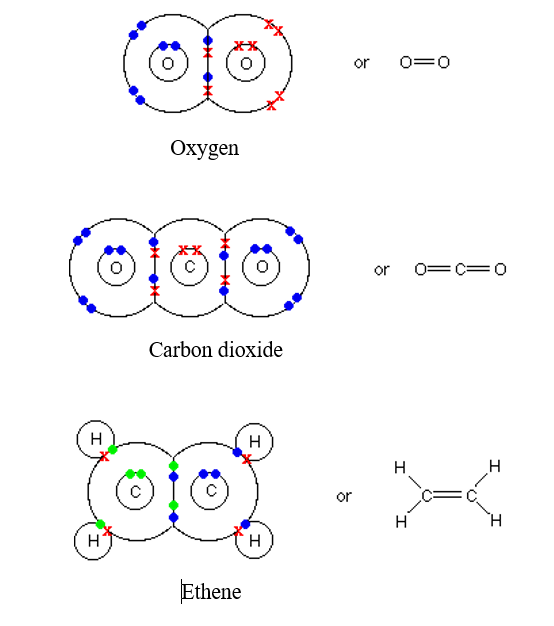
Ethene, C2H4:
Ethene forms a double covalent bond between two carbon atoms. As shown in the above figure.
Ethene is made from hydrogen atom (1s1) and carbon atoms (1s2 2s2 2px 12py1).
Promotion of an electron:
The carbon atom doesn’t have satisfied unpaired electrons to form the required number of bonds. So it requires to donate one of the 2s2 pair to the empty orbit 2pz. The same thing happens with the formation of a carbon bond.
Now, this carbon atom is in an excited state.
The newly formed orbitals are known as sp2 hybrids because they are formed by a combination of one s and two p orbitals. These sp2 hybrid orbitals arrange themselves as far as they can i.e. at 120ᵒ to each other in a plane. The remaining p orbitals align themselves at right angles to them.
Ethene, C2H4
Ethene is comprised of four hydrogen atoms and two carbon atoms and before joining of these molecules, the arrangement would look like the form given below:
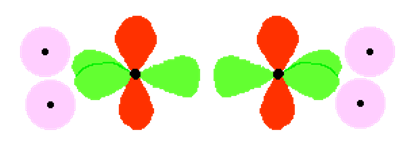
The different atomic orbitals that are pointing towards each other will now merge to give molecular orbitals. Each orbit will contain a bonding pair of electrons. The molecular orbitals that are formed by end-to-end overlapping of atomic orbitals are known as sigma bonds.
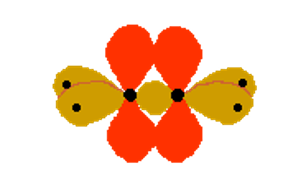
In the above diagram, the black dots are representing the nuclei of the atoms.
It is also noticed that the p orbitals are very close to each other and they are overlapping sideways.
This sideway overlapping creates a molecular orbital but it is of a different kind. Here the electrons aren’t held on a line between the two nuclei, above and below the molecule. A formation of bond in this way is called a pi bond.
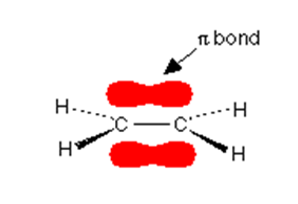
Note:
The sigma bond is represented by using the lines – and each line sows one pair of shared electrons. The other lines represent the direction of the bond in it. In a pi region, two electrons make up a bond and these two electrons can move freely within that space.
References:
https://www.chemguide.co.uk/atoms/bonding/doublebonds.html
https://en.m.wikipedia.org/wiki/Carbon%E2%80%93carbon_bond
https://study.com/academy/lesson/double-bond-definition-formation-example.html
https://en.wikipedia.org/wiki/Covalent_bond
