Introduction
What is a chemical bond?
A Chemical bond detains molecules together. It also creates temporary connections that are essential to life.
What is chemical bonding?
Chemical bonding is any relationship that is responsible for the association of atoms into molecules, ions, crystals and other species that makes up the substance of the everyday world. When atoms approach each other, their nuclei and electrons interact and tend to arrange themselves in space in such a way that their net energy is lower than it would be in any other arrangement. If the net energy of a group of atoms is lower than the sum of energies of the component atoms, they then bond with each other and the energy that is lowering is the bond energy.
Types of chemical bonds:
The different types of bond include:
- Ionic bond
- Covalent bond
- Dative bond
- Hydrogen bond
- London dispersion forces
What is the dative bond?
A dative bond is also termed as Coordinate Covalent bond. it is that type of chemical bond in which one atom provides a shared pair of electron for the formation of a bond.
OR
A chemical bond that is formed between two atoms due to sharing of the electron pair in which only one atom provides a shared pair of electron for bond formation.
In a formation of a dative bond, other atom does not provide electron for sharing. It is one-sided sharing. The formation of the coordinate covalent bond belongs to the atoms that have lone pairs of electrons. the contributions of electrons towards combining the atoms in a covalent bond are generally equal. The atom that provides electron pair is termed as Donor and the other which takes the electron pair is called Acceptor.
Representation:
Dative bond is represented by an arrowhead that points from donor atom to the acceptor.
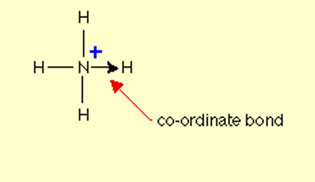
Explanation:
Dative bonds play a significant role in life’s most critical processes. For example, oxygen is transferred throughout our body via the heme group, an iron-containing coordination compound present in the blood cell.
Ionic compounds are composed of one or more cations (positive ions) or anions (negative ions) that are bonded together because of the opposite charges. The total charge on the compound is zero as positive and negative charges balance each other.
The coordinate compounds are little more complicated ionic compounds. One of the ions has to be a complex ion in a coordination compound. It is not difficult to identify which is a complex ion as it looks complex. For example:
[Pt(NH3)3 Br]+ and Cl–
As the above example show that chloride ions are showing a simple ion while the first one is showing the complex ion.
A complex ion is composed of two things, a metal ion and compounds called ligands. The ligands are neutral molecules or ions that contain lone pairs of electrons that can bond with the metal ion. Common ligands are ammonia (NH3), water (H2O) and halide ions (Cl–, Br–etc).
Ligands are considered Lewis bases because they share their electron pairs with the metal ion. As metal ions are always positive so they are attracted to lone pairs of electrons. The resulting bond between the metal ion and the ligands are termed as dative or coordinate covalent bond.
Examples of a coordinate covalent bond:
- Ammonia and Hydrogen Chloride:
When ammonia and hydrogen chloride are allowed to mix, solid ammonium chloride is formed with a cloud of dense white smoke. The reaction is given as:
NH3 (g) + HCl (g) ® NH4Cl (s)
Ammonium ions, NH4+ are created by the transfer of a hydrogen ion (proton) from the hydrogen chloride molecule to the lone pair of electrons on the ammonia molecule.
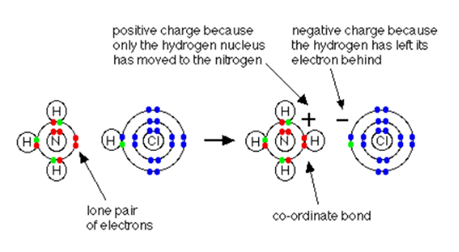
When the ammonium ion, NH4+ is created, the fourth hydrogen attached itself to a dative bond because only the nucleus of hydrogen is transferred from the chlorine to the nitrogen. The electron of the hydrogen is left behind on the chlorine to form a negatively charged chloride ion. Once the ammonium ion is formed, it is very difficult to tell the difference between the coordinate covalent bond and the ordinary covalent bonds. In the diagram below, though the electrons are represented differently, in reality, there is no difference between them.
- The reaction between ammonia and boron trifluoride:
Boron trifluoride is a compound that does not have a noble gas structure around the boron atom.in a bonding level, the boron only has three pairs of electrons. On the other hand, there is a place for four pairs. BF3 itself is electron deficient. There is a lone pair on the nitrogen of an ammonia molecule that can be used to overcome that deficiency and a compound will be formed that includes coordinate bond.
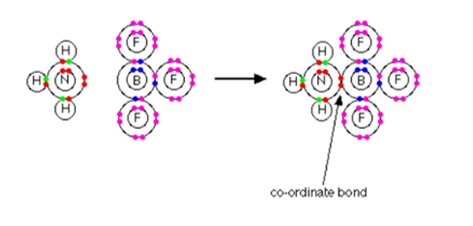
Lines are used to representing the bonds; in a simple form they can be drawn as:
The nitrogen end of the bond has become positive because the electron pair has moved away from the nitrogen towards the boron which has then become negative.
- Aluminum Chloride Structure:
At a temperature of about 180 ᵒC, the aluminium chloride sublimes. Because of the strong attraction between positive and negative ions, it would have very high melting and boiling points if it only contains ions. This implies that when it sublimes at a relatively low temperature, it must be covalent. The below diagram shows the dots and crosses of the out electrons only.
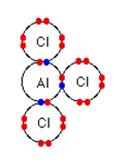
Lewis Dot Diagram of AlCl3
Aluminium chloride is an electron-deficient. There is a similarity between AlCl3 and BF3 because aluminium and boron belong to the same group of the periodic table.
The measurements of the relative formula mass of aluminium chloride describe that its formula in the vapor at the sublimation temperature is not AlCl3 but Al2Cl6. It exists as a dimer i.e. in which two molecules joined together. Using lone pairs on the chlorine atoms, the bonding between the two molecules is coordinate. Each of the chlorine atoms has three lone pairs but only the two are shown below:
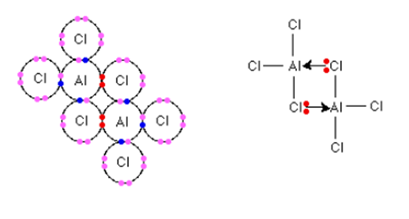
When two coordinate bonds are formed, the energy is released. Hence the dimer is more energetically stable than the two separate AlCl3 molecules.
The bonding in Hydrated Metal ions:
As water molecules are strongly attracted to ions in solution, the water molecules clustered themselves around positive or negative ions. In most cases, the attractions are very considerable that formal bonds are created, and this is true for almost all positive metal ions. Ions having water molecules attached are termed as hydrated ions.
Although aluminium chloride is a covalent compound, when it dissolves in water ions are produced. Six molecules of water are bond to aluminium to give an ion having a formula of Al(H2O)63+. It is termed as the hexaaquaaluminum complex ion having six (Hexa) water molecules (aqua) enclose around an aluminium ion. Here the bonding is coordinate by using lone pairs on the water molecules.
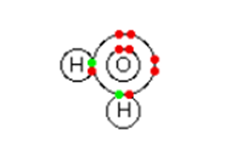
Figure showing water has two lone pairs of electrons
The electron configuration of aluminum is 1s22s22p63s23px1. When an Al3+ ion is formed, it loses the 3-level electrons to leave 1s22s22p6. This tells us that all the 3-level orbitals are now empty. The aluminium reorganizes (hybridizes) six of these (the 3s, three 3p, and two 3d) to produce six new orbitals all with the same energy. The six hybrid orbitals that are formed accept lone pairs from six water molecules.
You might wonder why it chooses to use six orbitals rather than four or eight or whatever. Six is the maximum number of water molecules it is possible to fit around an aluminium ion (and most other metal ions). By making the maximum number of bonds, it releases most energy and is the most energetically stable.
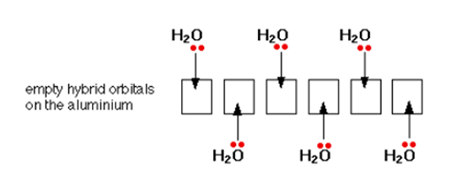
On each of the water molecules, only one lone pair is shown. The other lone pair is pointing away from the aluminium and hence it is not involved in the bonding. The resulting ion looks like the figure given below:
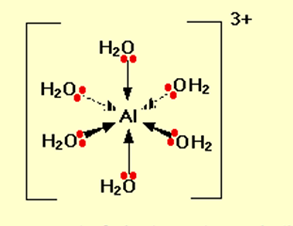
Because of the movement of electrons towards the center of the ion, the 3+ charge is no longer located entirely on the aluminium but is now spread over the whole of the ion.
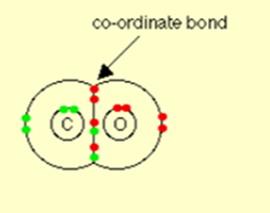
Carbon monoxide, CO:
Carbon monoxide is considered as having two ordinary covalent bonds between the carbon and the oxygen in addition to It coordinate bond using a lone pair on the oxygen atom.
Nitric Acid:
In this example, one of the oxygen atoms can be thought of as attaching to nitrogen via a co-ordinate bond using the lone pair on the nitrogen atom.
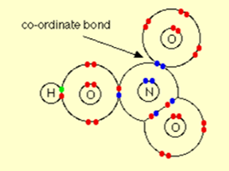
The above structure is misleading as it suggests that the two oxygen atoms on the right-hand side of the diagram are joined to the nitrogen in different ways. As both the bonds are identical in length and strength hence the arrangement of the electrons must be identical. There is no other way of representing this by using dots and cross pictures. The bonding involves delocalization.
Formation of the Hydronium ion:
H2O(l) + H+ (aq) [H3O] +
The above reaction shows that a pair of electrons (a lone pair) from the oxygen atom of the water is donated to a proton to form an oxygen-hydrogen dative bond in the oxonium ion.
The reaction occurs whenever you dissolve a soluble acidic substance in water, but the proton can also come from another water molecule in a self-ionization process.
2H2O (l) ⇌ H3O+ + OH– (aq)
References:
https://study.com/academy/lesson/coordinate-covalent-bond-definition-examples.html
https://www.citycollegiate.com/hybridization11.htm
https://www.chemguide.co.uk/atoms/bonding/dative.html
https://www.brainkart.com/article/Co-ordinate-covalent-bonding-or-Dative-bonding_2761/
