Definition
According to Dalton’s theory atom is smallest particle which could not be divided any further. Atom is the entity that take part in a chemical reaction. For example, He and Ne, etc. have atoms, which exists independently. While atoms of hydrogen, nitrogen and oxygen do not exist independently.
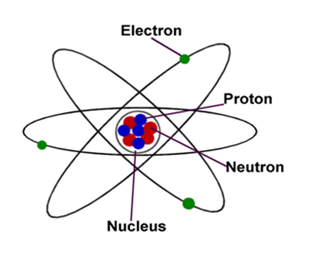
An atom is further composed of subatomic particles like electron, proton, neutron, hypron, neutrino, anti-neutrino etc. more than 100 such particles are exist in an atom. However, Electron, Proton and Neutron are regarded as fundamental particles. An atom is composed of two regions: the nucleus, the center of atom contain proton and neutron, and the outer portion of the atom holds electrons in its orbit around the nucleus [1].
Ion
Ions are those species which have a positive or a negative charge.
Whenever an atom of an element loses one or more electrons, positive ions are formed. A sufficient amount of energy is required to a neutral atom to ionize it.
A → A+ + e–
This A+ is called a cation.
A cation may carry +1, +2, +3, etc. charge or charges. Formation of positive ions is an endothermic process. The most common positive ions are formed by the metal atoms are Na+, K+, Ca+, Mg+, Al+ etc.
When a neutral atom picks up one or more electrons, a negative ion is produced, which is called an anion.
B + e– → B–
Energy is released when an electron is introduced into an isolated atom. This is an exothermic process. The most common negative ions are F–, Cl–, Br–, S2- etc.
Atomic Mass Unit
It is defined as a unit of mass which is used to express atomic weight, it is equal to 1/12th of the mass of carbon-12.
Orbital
It is the region in the atom where an electron is likely to be found.
Subatomic Particles
Proton:
Proton is subatomic particle present in the center of atom making up nucleus. It has positive charge. The number of proton in an element’s nucleus is known as atomic number. In 1886, German physicist, E. Goldstein discovered positive rays through discharge tube provided with a perforated cathode. He projected alpha particles on gold foil so the positive alpha particles were deflected. He said that protons exist in the nucleus and have positive charge.
Properties
- They are deflected by an electric as well as magnetic field showing that these are positively charged.
- The e/m value for positive rays is always smaller than that of electron and also depend upon nature of gas used in the discharge tube.
- These rays travel in the straight line in a direction opposite to the cathode rays (electron).
Neutron
The neutron is also subatomic particle having no net electric charge. Neutron is present in nucleus along with proton. It is almost similar in mass to a proton. Chadwick discovered neutron in 1932. Neutron determine the isotope of an atom and sometime stability of an atom.
Properties
- Neutron do not ionize gases.
- When neutrons are used as projectiles, they can carry out the nuclear reactions.
- Neutrons are extremely penetrating particles.
- They can expel high speed protons from water, paper and cellulose.
Electron
An electron is negatively charged subatomic particle. It is present outside of and surrounding the nucleus. An electron has a small mass as compared to neutron or proton, it carries negative charge (1.602 X 10-19 coulomb). Electron was discovered in 1897 by J. J. Thomson. Electrons can abbreviated as e–.
Properties
- The electron has an intrinsic angular momentum.
- Electron can ionize gases.
- It has no known substructure.
| Particle | Charge (Coulomb) | Relative charge | Mass (kg) | Mass (amu) |
| Proton | +1.6022 x 10 -19 | +1 | 1.6726 x 10 -27 | 1.0073 |
| Neutron | 0 | 0 | 1.6750 x 10 -27 | 1.0087 |
| Electron | -1.6022 x 10 -19 | -1 | 9.1095 x 10 -31 | 5.4858 x 104 |
Other Basic Atomic Particles
Alpha Particles
They can be denoted by He2+, α2+ or α. They are helium nuclei and consist of two protons and two neutrons. Alpha particles are produced by alpha decay of an atom through which they become a new element. This process occur in those elements which have large radioactive nuclei. They are not harmful. Alpha decay process can be easily stopped through a sheet of paper or by skin. This process is mostly used in artificial heart pacemakers and space probes [4].
Beta Particles
They are known as free electrons or positrons, having high energy and high speed. They are released as a result of beta decay. Positrons have the same mass as electrons but are positively charged. They have 100 times more penetrating power than alpha particles. Their emission can be stopped by household items like wood, plate or sheet. Beta particles are used in radiation to treat cancer.
Beta– (β–) or Electron Emission
They can be produced when many neutrons make the nucleus of an atom unstable. Neutrons are decay and produced protons, an electron and an anti-neutrino. Proton stay remains in the nucleus and electron and anti-neutrino are released. The electron is called beta particle.
Beta+ (β+) or positron Emission
When excess of proton make the nucleus of an atom unstable. During the process proton is converted into neutron, a positron and a neutrino. Neutron remain stay in the nucleus but the positron and the neutrino are released.
Atomic Number
The number of proton in the nucleus of each atom of an element. The letter ‘’Z’’ represents the number.
For example: The atomic number of nitrogen is 7. This means that nitrogen has 7 proton and 7 electron.
Mass number
It is total number of proton and neutron present in the nucleus of each atom of an element.
Mass number = No. of proton + no of neutrons
= atomic number – no of neutron
For example: the mass number of fluorine is 19 and atomic number is 9. Thus the number of neutron in an atom of fluorine is 19-9 =10.
Isotopes
Definition
The element which exist in different forms, having same atomic number but different mass number. The isotopes that cannot decay during a defined period are called stable isotopes and the isotopes that can decay during a defined period are called unstable or radioactive isotopes [2].
For example:
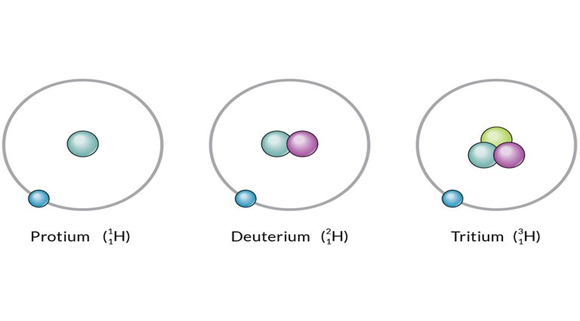
- Isotopes of Hydrogen: There are three isotopes: protium, deuterium and tritium. Each has 1 proton and 1 electron but the number of neutrons is different. Tritium is radioactive isotope of hydrogen.
- Isotopes of Carbon: It has two stable isotopes 12C and 13C and one radioactive isotope 14C.
- Isotopes of Chlorine: There are two isotopes of chlorine 35Cl and 37Cl.
- Isotopes of Uranium: There are three isotopes of uranium 234U, 235U and 238U.
Quantum Numbers
These are the parameters that describe the distribution of electrons: these are as follows [3].
- Principal Quantum Number:
They represents the main energy level or shells occupied by the electrons, the value of n represents the shell or energy level in which the electrons are revolves around the nucleus. Letter notations K, L, M, N, etc are also used to denote the various shells.
For example, when n=1, it is called K shell, for n=2 it is called L shell and so on.
The value of n also determine the location of electron in an atom, i.e the distance of electron from the nucleus, greater the value of ‘n’ greater will be distance of electron from the nucleus. It is quantitative measure of the size of an electronic shell. ‘n’ also provides us the energy of electron in a shell.
- Secondary Quantum Number:
They represents the energy sublevels occupied by the electrons. The values (l) are:
l= 0,1,2, 3,…………………..(n-1)
Its value depend upon n. These values represents different subshells, which are designed by small letters, s, p, d, f. They stand for sharp, principal, diffused and fundamental, respectively.
A subshell may have different shapes depending upon the value of l.
l=0 s- subshell spherical
l=1 p subshell dumb-bell
l=2 d=subshell complicated shape
- Magnetic Quantum Number
They represents the possible orientation in 3-D space of each type of orbital.
Its values are m= 0, +1, +2, +3, …………………..
The value of ‘m’ depends upon value of ‘l’. (2l+1).
- Spin Quantum Number
They represent that in the presence of magnetic field electron can have two possible orientation. Only two electrons can occupy the same orbital.
Electronic distribution
An orbital like s, px, py, pz and dxy etc. can have at the two most two electrons.
The maximum number of electrons that can be accommodate in a shell is given by 2n2 formula where n is principle quantum number and it cannot have zero value. Following are the rules that have been adopted to distribute the electrons in subshells or orbitals.
- Aufbau principle
- Pauli’s exclusion principle
- Hund’s rule
The arrangement of subshells in ascending order of their energy may be as follows: 1s,2s,2p, 3s,3p,4s,3d,4p,5s,4d,5p.6s,4f,5d,6s,4f,5d,6p,7s and so no.
Aufbau principle
The electrons should be filled in energy subshells in order of increasing energy value. The electrons are first placed in 1s, 2s, 2p and so on.
Pauli’s exclusion principle
Two electrons in the same orbital should have opposite spin.
Hund’s Rule
If degenerate orbitals are available and more than one electrons are to be placed in them, they should be placed in separate orbitals with the same spin rather than putting them in the same orbital with opposite spins.
For example: C = 1s 2s 2px 2py
References
- https://courses.lumenlearning.com/introchem/chapter/overview-of-atomic-structure/
- https://en.wikiversity.org/wiki/Atomic_structure
- https://personal.utdallas.edu/~scortes/ochem/OChem1_Lecture/Class_Materials/02_atomic_structure.pdf
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Atomic_Theory/The_Atom/Sub-Atomic_Particles
